추천 제품
Quality Level
분석
97%
mp
89-91 °C (lit.)
작용기
imide
maleimide
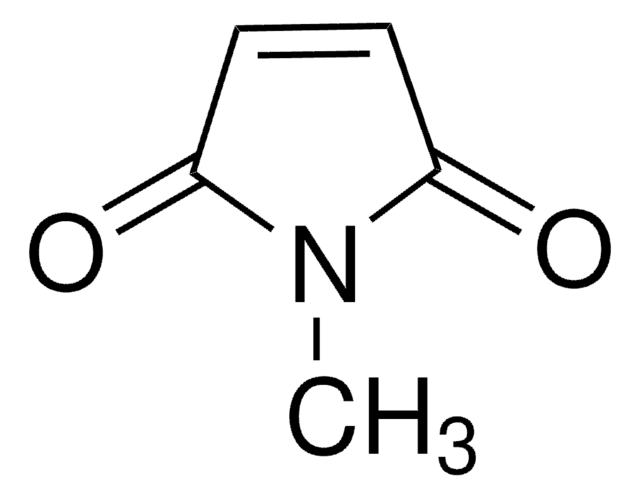
SMILES string
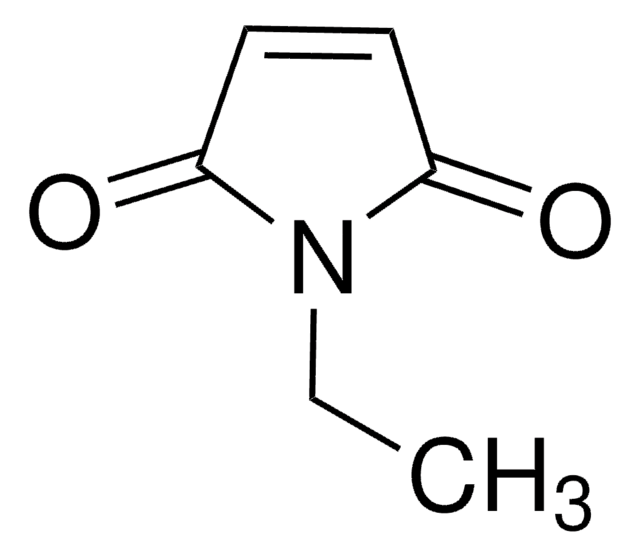
O=C1C=CC(=O)N1C2CCCCC2
InChI
1S/C10H13NO2/c12-9-6-7-10(13)11(9)8-4-2-1-3-5-8/h6-8H,1-5H2
InChI key
BQTPKSBXMONSJI-UHFFFAOYSA-N
일반 설명
N-Cyclohexylmaleimide (CHMI, NCMI) is a cyclic imide. Diels-Alder reactions of 9-hydroxymethylanthracene with CHMI catalyzed by hydrophobic nanospace confined within the self-assembled Pd6 open cage bearing triimidazole walls have been reported. It undergoes Diels-Alder reactions with various aromatic hydrocarbons promoted by self-assembled coordination cage. This cage acts as nanometer-sized molecular flask and hence promotes this reaction. CHMI is reported to undergo radical polymerization readily under various polymerization conditions to afford poly(CHMI), having excellent thermal stability.
애플리케이션
N-Cyclohexylmaleimide (CHMI, NCMI) may be used for the preparation of hyperbranched copolymers of p-(chloromethyl)styrene (CMS) and NCMI, via atom transfer radical copolymerization reaction.
N-Cyclohexylmaleimide contaminated with N-cyclohexylmaleamic acid (less than 0.9 wt%) may be used for the heat-resistant poly(methyl methacrylate) resin free from coloration and for methyl methacrylate homopolymer. It may be used as monomer in the preparation of styrene/N-cyclohexylmaleimide copolymers with small polydispersities and controlled molecular weights, via free radical copolymerization. It may be used in the synthesis of hyperbranched copolymers by the atom transfer radical copolymerization with p-(chloromethyl)styrene catalyzed by CuCl/2,2′-bipyridine in cyclohexanone or anisole.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Aquatic Acute 1 - Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1A - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Shinnosuke Horiuchi et al.
Chemistry, an Asian journal, 6(7), 1839-1847 (2011-02-22)
A self-assembled coordination cage serves as a nanometer-sized molecular flask to promote the Diels-Alder reactions of aromatic hydrocarbons with N-cyclohexylmaleimide. The coordination cage accelerated the Diels-Alder reaction of anthracene at the electronically unfavorable, terminal benzene ring to give a compact
Increase in thermal stability of vinyl polymers through radical copolymerization with N-cyclohexylmaleimide.
Otsu T, et al.
Polymer International, 25(3), 179-184 (1991)
Living free radical donor-acceptor copolymerization of styrene and N-cyclohexylmaleimide and the synthesis of poly [styrene-co-(N-cyclohexylmaleimide)]/polystyrene block copolymers.
Schmidt-Naake G and Butz S.
Macromolecular Rapid Communications, 17(9), 661-665 (1996)
Bingchuan Wei et al.
Journal of chromatography. A, 1526, 104-111 (2017-10-29)
Reversed-phase liquid chromatography (RPLC) has been commonly used in IgG2 disulfide isoforms analysis. Recently, the columns packed with large pore superficially porous particles (SPP) have become available commercially. This work explores the application of this SPP technology in IgG2 disulfide
Hyperbranched copolymers of p-(chloromethyl) styrene and N-cyclohexylmaleimide synthesized by atom transfer radical polymerization.
Jiang X, et al.
Journal of Applied Polymer Science, 78(11), 1992-1997 (2000)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.