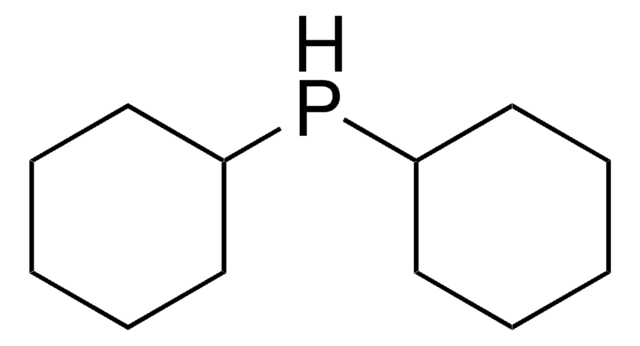
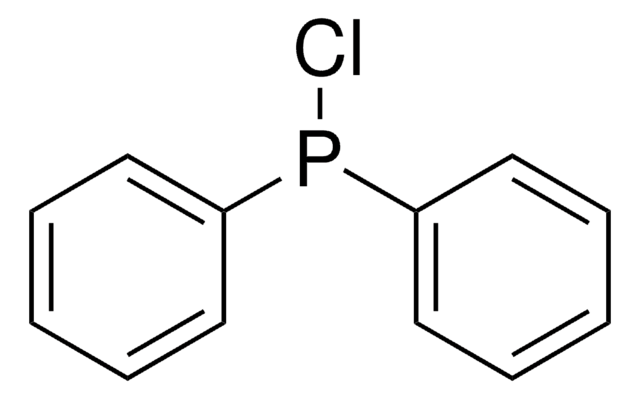
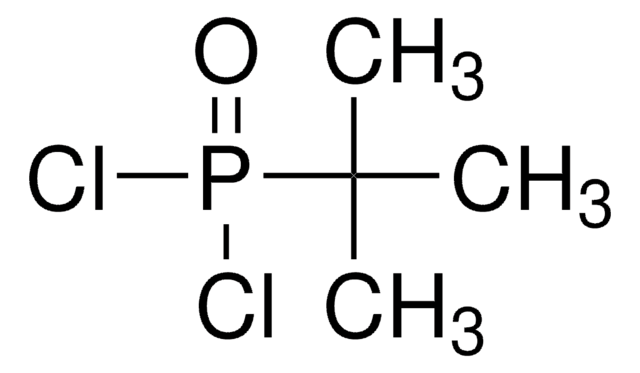
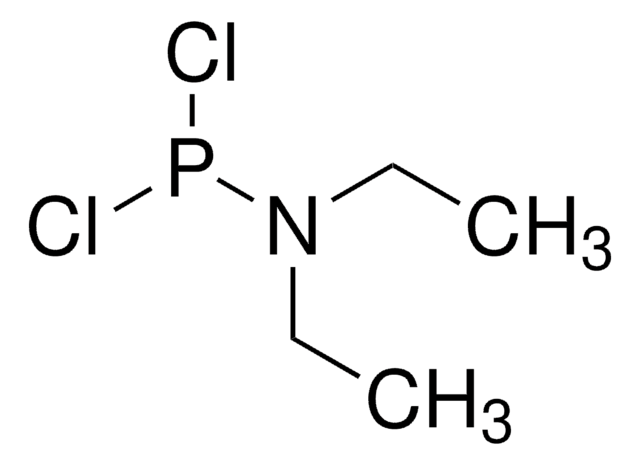
추천 제품
분석
98%
반응 적합성
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
mp
44-49 °C (lit.)
작용기
phosphine
SMILES string
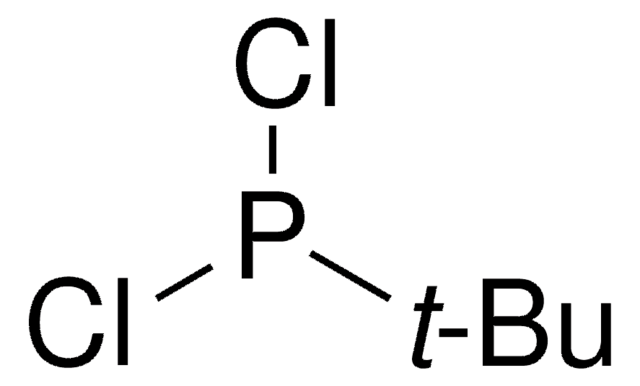
CC(C)(C)P(Cl)Cl
InChI
1S/C4H9Cl2P/c1-4(2,3)7(5)6/h1-3H3
InChI key
NMJASRUOIRRDSX-UHFFFAOYSA-N
애플리케이션
tert-Butyldichlorophosphine can be used as a reactant for the synthesis of:
- Dihydrobenzooxaphosphole core.
- tert-Butyl functionalized 1,3-C6H4(CH2PR2)2 (PCP) pincer ligands.
- Chloro-phosphinite reagent by reacting with sodium ethoxide.
- 2-(tert-Butylhydrophosphoryl)-1-phenyl-1H-imidazole derivatives.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
보충제 위험성
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Solid-phase synthesis and catalytic screening of polystyrene supported diphosphines.
Samuels MC, et al.
Topics in Catalysis, 59(19-20), 1793-1799 (2016)
Synthesis of P-Chiral Dihydrobenzooxaphosphole Core for BI Ligands in Asymmetric Transformations.
Li G, et al.
The Journal of Organic Chemistry, 82(10), 5456-5460 (2017)
Synthesis and coordination chemistry of new asymmetric donor/acceptor pincer ligands, 1, 3-C6H4 (CH2PtBu(Rf))2 (Rf= CF3, C2F5).
Debnath S, et al.
Dalton Transactions, 47(35), 12420-12430 (2018)
Imidazolio-substituted secondary phosphine oxides as potential carbene reagents.
Chang YC, et al.
Polyhedron, 100(19-20), 382-391 (2015)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.