추천 제품
분석
96%
양식
liquid
반응 적합성
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
refractive index
n20/D 1.482 (lit.)
bp
48 °C/3 mmHg (lit.)
density
0.951 g/mL at 25 °C (lit.)
작용기
phosphine
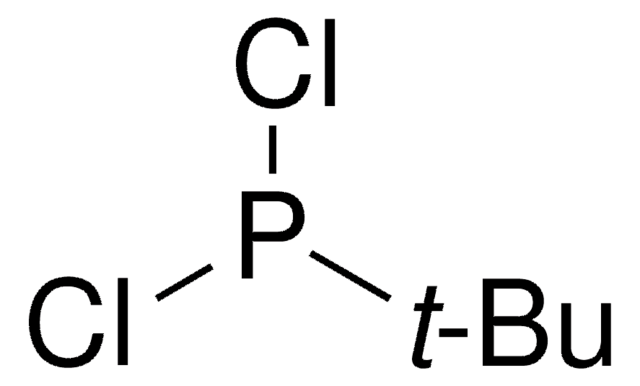
SMILES string
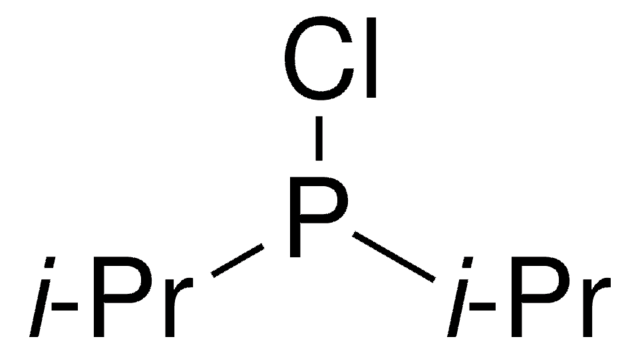
CC(C)(C)P(Cl)C(C)(C)C
InChI
1S/C8H18ClP/c1-7(2,3)10(9)8(4,5)6/h1-6H3
InChI key
MCRSZLVSRGTMIH-UHFFFAOYSA-N
일반 설명
Di-tert-butylchlorophosphine belongs to the class of phosphine ligands. It is used for cross-coupling reactions because of the flexibility of its electronic and steric properties. It plays a key role in stabilizing and activating the central metal atom and is used in reactions such as transition metal-catalyzed C-O, C-N, and C-C bond-forming reactions.
애플리케이션
Di-tert-butylchlorophosphine can be used as a ligand in:
- The Pd-catalyzed amination reaction with aryl halides.
- The Pd-catalyzed Suzuki-Miyaura cross-coupling of arylboronic acids with aryl bromides and chlorides.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
143.6 °F - closed cup
Flash Point (°C)
62 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
Jesudoss V Kingston et al.
The Journal of organic chemistry, 72(8), 2816-2822 (2007-03-24)
Pro-azaphosphatrane 1a [P(iBuNCH2CH2)3N] reacts with iodine under mild conditions to give [IP(iBuNCH2CH2)3N]I in excellent yield, which on subsequent reaction with ammonia followed by deprotonation with KOtBu provided HN=P(iBuNCH2CH2)3N (3a) in quantitative yield. Reaction of 3a with R'2PCl afforded sterically bulky
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.