추천 제품
양식
liquid
Quality Level
반응 적합성
reaction type: C-C Bond Formation
농도
0.5 M in toluene
density
0.927 g/mL at 25 °C
SMILES string
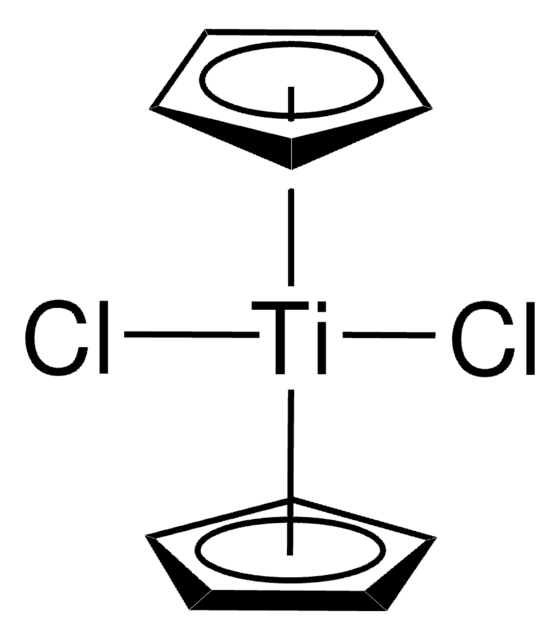
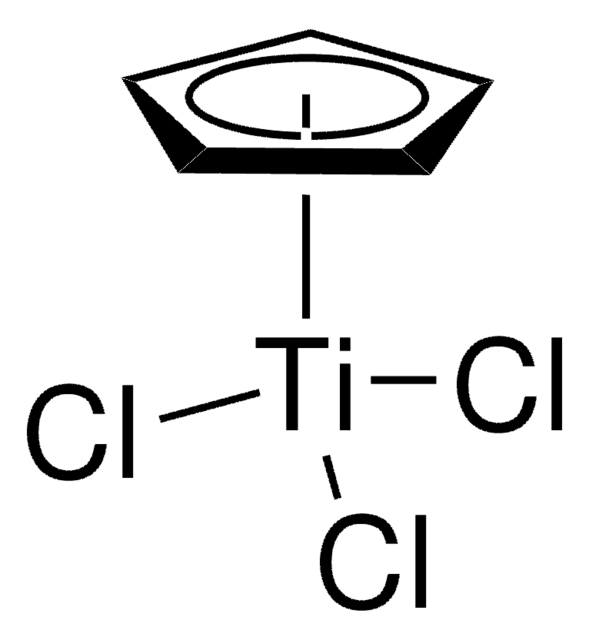
[CH]1[CH][CH][CH][CH]1.[CH]2[CH][CH][CH][CH]2.C[Al](C)C[Ti]Cl
InChI
1S/2C5H5.2CH3.CH2.Al.ClH.Ti/c2*1-2-4-5-3-1;;;;;;/h2*1-5H;2*1H3;1H2;;1H;/q;;;;;;;+1/p-1
InChI key
QEJAQNUJXFLWSP-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- For the conversion of carbonyl groups of chlorophyll derivatives into the corresponding exo-methylene (or vinylidene) groups.
- In the synthesis of β-C-glycosides from 3-OH glycol esters.
- As a versatile methylenation reagent for the conversion of ketones and aldehydes to olefins. It offers facile reaction with hindered ketones and allows the conversion of esters to vinyl ethers.
- To olefinate aldehydes.
- To methylenate a chiral polyhydroxyketone with high diasteroselctivity.
포장
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 3 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1B - STOT RE 2 - STOT SE 3
표적 기관
Central nervous system, Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
39.2 °F - closed cup
Flash Point (°C)
4 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
문서
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.