모든 사진(3)
About This Item
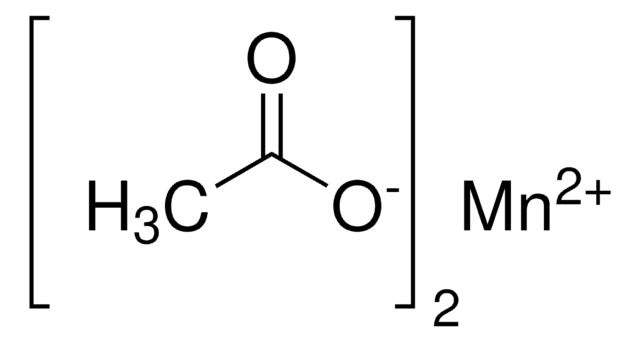
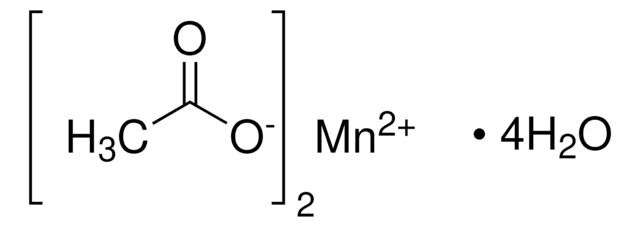
Linear Formula:
(CH3COO)3Mn · 2H2O
CAS Number:
Molecular Weight:
268.10
Beilstein:
3732626
EC Number:
MDL number:
UNSPSC 코드:
12352103
PubChem Substance ID:
NACRES:
NA.23
추천 제품
Quality Level
분석
97%
양식
powder or chunks
반응 적합성
core: manganese
SMILES string
O.O.CC(=O)O[Mn](OC(C)=O)OC(C)=O
InChI
1S/3C2H4O2.Mn.2H2O/c3*1-2(3)4;;;/h3*1H3,(H,3,4);;2*1H2/q;;;+3;;/p-3
InChI key
ONJSLAKTVIZUQS-UHFFFAOYSA-K
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Manganese(III) acetate dihydrate is a coordination compound of manganese in the +3 oxidation state, exhibiting moderate solubility in water and acetic acid. It is used as a precursor for synthesizing manganese oxides and other manganese-containing materials. These materials have applications in batteries, catalysis, and other energy-related technologies. Additionally, it is also used as a mild and selective oxidizing agent in organic synthesis.
애플리케이션
Manganese(III) acetate dihydrate can be used as:
- A precursor in the synthesis of manganese oxide (Mn3O4) nanostructures, which are employed as anode materials in lithium-ion batteries.
- A manganese source in the sol-gel synthesis of Mn-doped ZnO thin films. The incorporation of manganese ions into the ZnO lattice is essential for modifying the electronic and optical properties of the films.
- A precursor for the synthesis of manganese oxide nanoparticles using a sol-gel process. These nanoparticles are evaluated for their performance in supercapacitor applications.
- A mild and selective oxidizing agent. Catalyzes allylic oxidation of a variety of alkenes in the presence of tert-butylhydroperoxide. Reagent used for radical cyclizations and α-keto-acetoxylation.
물리적 형태
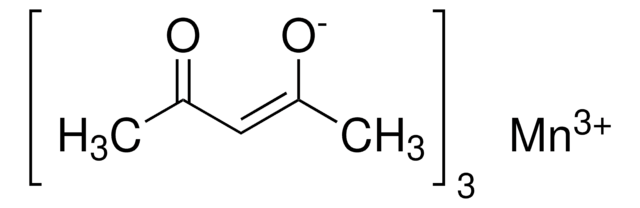
Crystallographic evidence suggests this material is an oxo-centered triangle of Mn(III) with bridging acetates having the molecular formula: Mn3O(OAc)9 · 6H2O.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
The Journal of Organic Chemistry, 62, 6978-6978 (1997)
Manganese(III)-Based Oxidative Free-Radical Cyclizations.
Barry B. Snider
Chemical reviews, 96(1), 339-364 (1996-02-01)
Demir, A.S. et al.
Tetrahedron, 55, 2441-2441 (1999)
Chemtracts Org. Chem., 403-403 (1991)
Tony K M Shing et al.
Organic letters, 8(14), 3149-3151 (2006-06-30)
Manganese(III) acetate catalyzed allylic oxidation of alkenes to the corresponding enones was investigated, showing excellent regioselectivity and chemoselectivity (functional group compatibility). Delta(5)-Steroids were transformed into bioactive Delta(5)-en-7-ones under a nitrogen atmosphere, whereas simple alkenes were converted into the corresponding enones
문서
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.