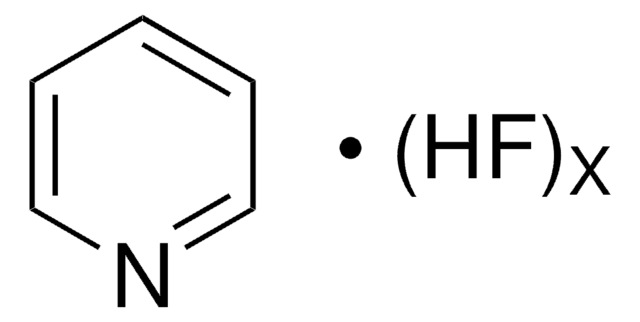
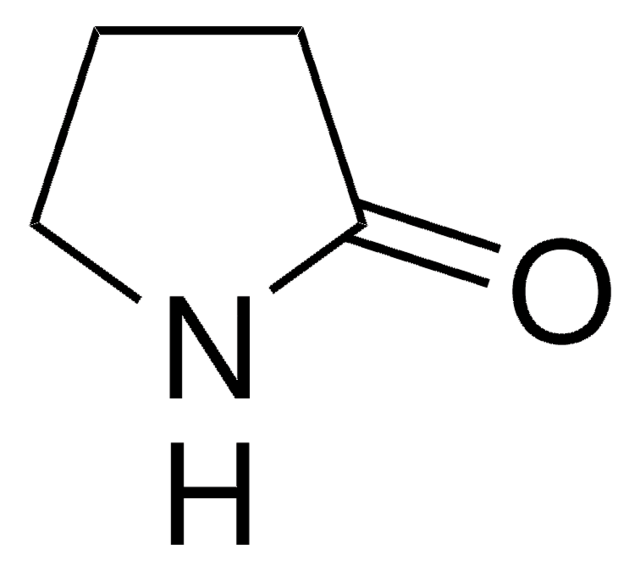
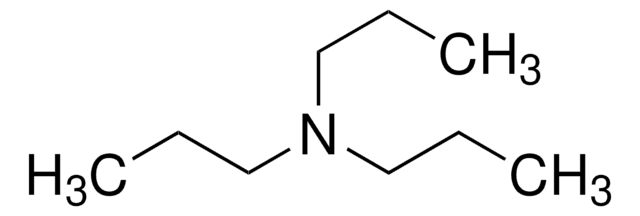
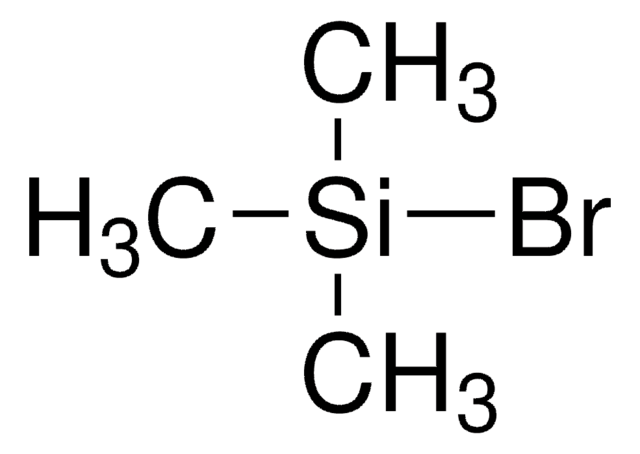추천 제품
Quality Level
분석
>95%
형태
solid
bp
192 °C (lit.)
mp
56 °C (lit.)
density
2.82 g/mL at 25 °C (lit.)
작용기
phosphine oxide
저장 온도
2-8°C
SMILES string
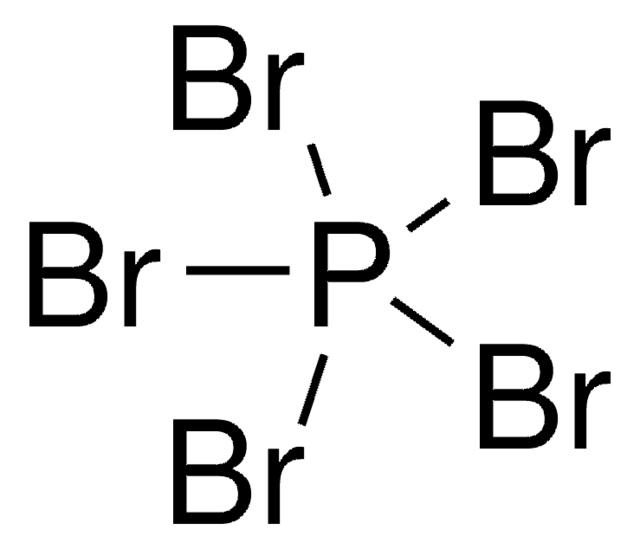
BrP(Br)(Br)=O
InChI
1S/Br3OP/c1-5(2,3)4
InChI key
UXCDUFKZSUBXGM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Reaction of phosphorus pentabromide with phosphorus pentoxide at elevated temperatures affords phosphorus(V) oxybromide. Its Raman spectrum has been studied and the molecule has been reported to exhibit point group symmetry C3v. Phosphorus(V) oxybromide (Phosphorus oxide bromide) participates in the preparation of 1:1 addition ionic compound having melting point at 154·0°C, via reaction with gallium tribromide. This ionic compound contains non-metal cation POBr2+.
애플리케이션
Phosphorus(V) oxybromide may be used as brominating reagent in the synthesis of 2,4,6-tribromopyrimidine. It may be used in the preparation of bromoheterocyclic compounds.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
보충제 위험성
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Phosphorus oxybromide as a brominating agent; bromopyrimidines.
D R V GOLDING et al.
The Journal of organic chemistry, 12(2), 293-293 (1947-03-01)
The possible existence of the cation POBr2+ and some properties of phosphorus oxybromide, gallium tribromide, and their ionic 1: 1 addition compound.
Greenwood NN and Worrall IJ.
J. Inorg. Nucl. Chem., 6(1), 34-41 (1958)
A facile halogenation of some hydroxyheterocycles using triphenylphosphine and N-halosuccinimide.
Sugimoto O, et al.
Tetrahedron Letters, 40(42), 7477-7478 (1999)
The Raman spectrum and the molecular structure of phosphorus oxybromide.
Gerding H and van Driel M.
Rec. Trav. Chim., 61(6), 419-424 (1942)
90. The heats of hydrolysis of some halides and oxyhalides of phosphorus.
Charnley T and Skinner HA.
Journal of the Chemical Society, 450-452 (1953)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
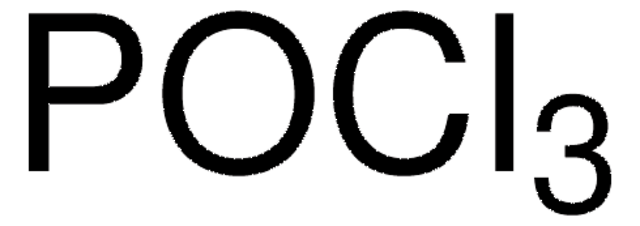

![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)