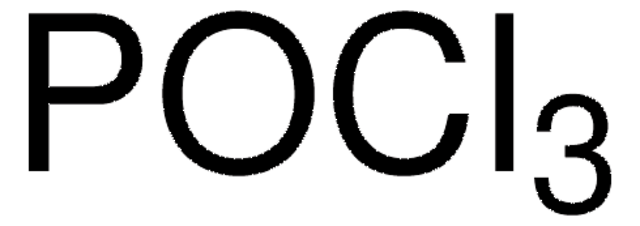모든 사진(3)
About This Item
Linear Formula:
PCl5
CAS Number:
Molecular Weight:
208.24
EC Number:
MDL number:
UNSPSC 코드:
12352300
PubChem Substance ID:
NACRES:
NA.21
추천 제품
Grade
reagent grade
Quality Level
vapor pressure
<1 mmHg ( 20 °C)
분석
95%
형태
powder
pH
1 (5 g/L)
mp
179-181 °C (subl.) (lit.)
SMILES string
ClP(Cl)(Cl)(Cl)Cl
InChI
1S/Cl5P/c1-6(2,3,4)5
InChI key
UHZYTMXLRWXGPK-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Phosphorus pentachloride is a light yellow colored phosphorous halide. It participates in the synthesis of thiazoline analogs.
애플리케이션
Phosphorus pentachloride may be used in the synthesis of following compounds:
Phosphorus pentachloride may be used as halogenating reagent to convert any substituted aldehyde into the corresponding vinyl halide. It can also undergo reaction with ammonium chloride to yield linear phosphazenes and cyclophosphazenes.
- benzenesulfonyl chloride
- malonic dinitrile
- aromatic sulfonyl chlorides
Phosphorus pentachloride may be used as halogenating reagent to convert any substituted aldehyde into the corresponding vinyl halide. It can also undergo reaction with ammonium chloride to yield linear phosphazenes and cyclophosphazenes.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT RE 2 Inhalation
표적 기관
Respiratory Tract
보충제 위험성
Storage Class Code
6.1B - Non-combustible, acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 1
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Ley.VS
Comprehensive Organic Functional Group Transformations II, 2 (1995)
Ley.VS et al.
Comprehensive Organic Functional Group Transformations: Synthesis: carbon with two heteroatoms, each attached by a single bond (2005)
Allcock H
Phosphorus-Nitrogen Compounds: Cyclic, Linear, and High Polymeric Systems (2012)
Eagleson M.
Concise Encyclopedia Chemistry, 120-120 (1994)
Synthetic studies of thiazoline and thiazolidine-containing natural products-1. Phosphorus pentachloride-mediated thiazoline construction reaction.
Ino A and Murabayashi A.
Tetrahedron, 55(34), 10271-10282 (1999)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.