모든 사진(1)
About This Item
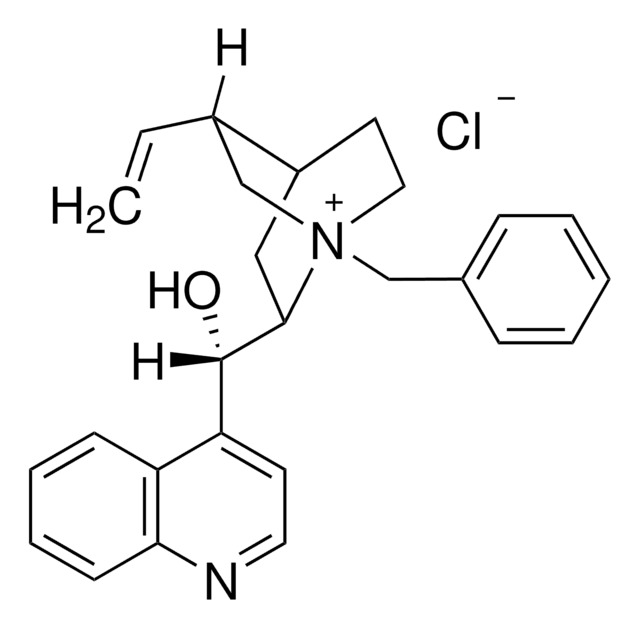
실험식(Hill 표기법):
C27H31ClN2O2
CAS Number:
Molecular Weight:
451.00
Beilstein:
5702637
MDL number:
UNSPSC 코드:
12352005
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
광학 활성
[α]20/D −235°, c = 1.5 in H2O
mp
200-205 °C (dec.) (lit.)
작용기
hydroxyl
phenyl
SMILES string
[Cl-].COc1ccc2nccc([C@@H](O)[C@@H]3C[C@@H]4CC[N@+]3(C[C@@H]4C=C)Cc5ccccc5)c2c1
InChI
1S/C27H31N2O2.ClH/c1-3-20-18-29(17-19-7-5-4-6-8-19)14-12-21(20)15-26(29)27(30)23-11-13-28-25-10-9-22(31-2)16-24(23)25;/h3-11,13,16,20-21,26-27,30H,1,12,14-15,17-18H2,2H3;1H/q+1;/p-1/t20-,21-,26-,27+,29+;/m0./s1
InChI key
JYDIJFKNXHPWBJ-GOGFHWEMSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
N-Benzylquininium chloride (or Quibec) belongs to the class of cinchona family of alkaloids. It is used as a catalyst in the presence of hydroxide bases in various phase transfer reactions, epoxidations, alkylations, and Michael reactions.
애플리케이션
N-Benzylquininium chloride can be used as a phase transfer catalyst:
- In the sulfenylation of various β-keto sulfoxides.
- To synthesize N-carbamoyl-protected β-nitroamines from α-amido sulfones by asymmetric aza-Henry reaction.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
Phase-Transfer-Catalyzed Asymmetric Aza-Henry Reaction Using N-Carbamoyl Imines Generated In Situ from ?-Amido Sulfones
Fini F, et al.
Angewandte Chemie (International ed. in English), 44(48), 7975-7978 (2005)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
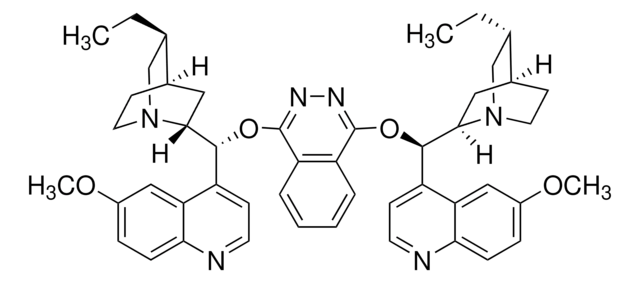
![N-[3,5-Bis(trifluoromethyl)phenyl]-N′-[(8a,9S)-6′-methoxy-9-cinchonanyl]thiourea 90%](/deepweb/assets/sigmaaldrich/product/structures/634/236/e688c89f-a93b-4698-a6fc-48e479a875cb/640/e688c89f-a93b-4698-a6fc-48e479a875cb.png)
![(11bS)-(+)-4,4-Dibutyl-4,5-dihydro-2,6-bis(3,4,5-trifluorophenyl)-3H-dinaphth[2,1-c:1′,2′-e]azepinium bromide](/deepweb/assets/sigmaaldrich/product/structures/230/279/5c1a7e7e-f791-4612-9920-56c6d7c4f735/640/5c1a7e7e-f791-4612-9920-56c6d7c4f735.png)
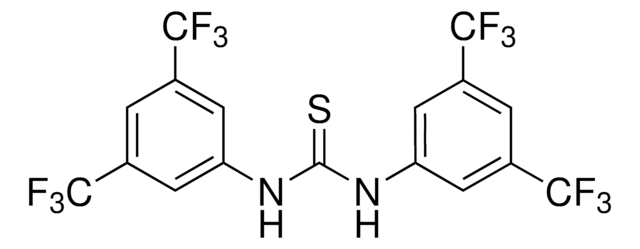
![1-[3,5-bis(trifluoromethyl)phenyl]-3-[(1R,2R)-(-)-2-(dimethylamino)cyclohexyl]thiourea AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/236/021/d944889d-2233-4700-9f2c-caa3652d0124/640/d944889d-2233-4700-9f2c-caa3652d0124.png)