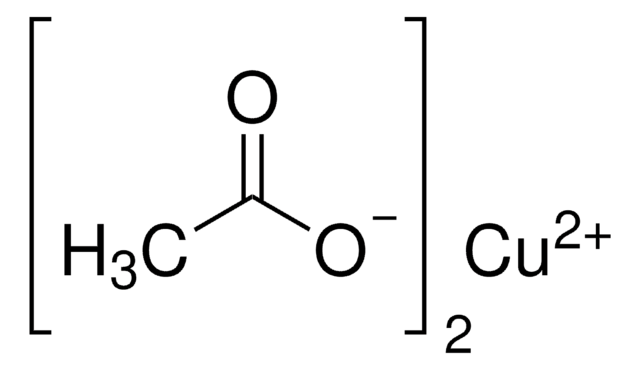
추천 제품
vapor density
6.8 (vs air)
Quality Level
분석
99.99% trace metals basis
양식
powder or crystals
반응 적합성
core: copper
환경친화적 대안 제품 특성
Catalysis
Learn more about the Principles of Green Chemistry.
sustainability
Greener Alternative Product
환경친화적 대안 카테고리
SMILES string
O.CC(=O)O[Cu]OC(C)=O
InChI
1S/2C2H4O2.Cu.H2O/c2*1-2(3)4;;/h2*1H3,(H,3,4);;1H2/q;;+2;/p-2
InChI key
NWFNSTOSIVLCJA-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Copper(II) acetate monohydrate is a binuclear copper complex significant in molecular magnetism, showing diamagnetic behavior at low temperatures and paramagnetic behavior at higher temperatures. It is soluble in water and has applications in sustainable energy storage, photovoltaics, and water purification. The compound can be synthesized by reacting acetic acid with copper(II) carbonate, hydroxide, or oxide, with large-scale production involving refluxing copper metal in air and acetic acid. Additionally, it can be used to form oxide nanoparticles through sonochemical methods.We are committed to bringing you Greener Alternative Products, which adhere to one or more of The 12 Principles of Green Chemistry. This product has been enhanced for catalysis. Click here for more information.
애플리케이션
Copper(II) acetate monohydrate can be used :
- To synthesize CuSbS2 nanoplates and a CuSbS2-Cu3SbS4 nanocomposite via hot injection method. CuSbS2 can be used as an absorber material in solar cells due to its favorable optical properties and direct band gap. The CuSbS2-Cu3SbS4 nanocomposite exhibits promising super capacitive properties, making it suitable for energy storage applications.
- To synthesize copper oxide nanoparticles (CuO NPs) using a green synthesis method involving psidium guajava leaf extract as both a reducing and capping agent. The CuO NPs exhibit excellent photocatalytic activity for degrading industrial dyes, such as Nile Blue (NB) and Reactive Yellow 160 (RY160). These nanoparticles can be used for purifying water resources contaminated with industrial dyes.
- As a copper precursor in synthesizing CuO semiconducting thin films via jet nebulizer spray pyrolysis technique, for P–N diode application. These CuO films find applications in supercapacitors, sensors, solar cells, photocatalysis and electrochromic devices.
특징 및 장점
- It is soluble in water makes a perfect precursor for the synthesis of new materials by sol-gel method
- With high purity of 99.99% (<150 ppm) and low heavy metals, it is ideal for Ir-catalyzed intramolecular C-H amination and the synthesis of carbinol.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
does not flash
Flash Point (°C)
does not flash
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Cu2ZnSnS4 films deposited by a soft-chemistry method.
Todorov T, et al.
Thin Solid Films, 517(7), 2541-2544 (2009)
Sonochemical synthesis and characterization of nanometer-size transition metal oxides from metal acetates.
Kumar RV, et al.
Chemistry of Materials, 12(8), 2301-2305 (2000)
Alexander Navarrete et al.
Faraday discussions, 183, 249-259 (2015-09-24)
A novel plasmonic reactor concept is proposed and tested to work as a visible energy harvesting device while allowing reactions to transform CO2 to be carried out. Particularly the reverse water gas shift (RWGS) reaction has been tested as a
Monica L Ohnsorg et al.
Langmuir : the ACS journal of surfaces and colloids, 31(22), 6114-6121 (2015-05-29)
Thin films can integrate the versatility and great potential found in the emerging field of metal-organic frameworks directly into device architectures. For fabrication of smart interfaces containing surface-anchored metal-organic frameworks, it is important to understand how the foundational layers form
Łukasz Orzeł et al.
Dalton transactions (Cambridge, England : 2003), 44(13), 6012-6022 (2015-02-28)
The nature of chlorophyll interactions with copper(II) ions varies considerably in organic solvents, depending on the dominant coordinative form. Besides formation of the metallo tetrapyrrolic complex, Cu(II) ions can cause oxidation of the pigment, reversible or irreversible, which can lead
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.