모든 사진(3)
About This Item
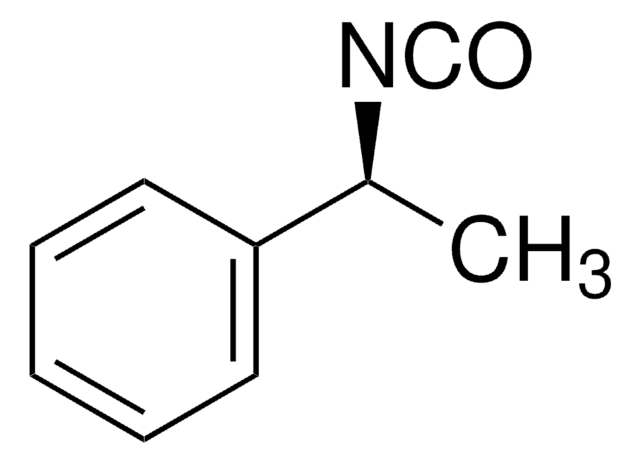
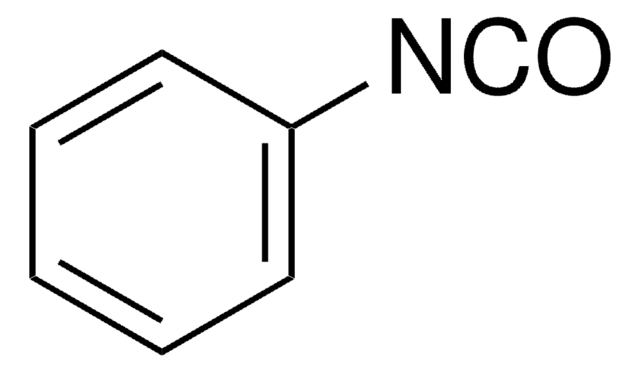
Linear Formula:
C6H5CH(CH3)NCO
CAS Number:
Molecular Weight:
147.17
Beilstein:
3196930
EC Number:
MDL number:
UNSPSC 코드:
12352118
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
99%
양식
liquid
광학 활성
[α]20/D +10°, neat
광학 순도
ee: 96% (GLC)
refractive index
n20/D 1.513 (lit.)
bp
55-56 °C/2.5 mmHg (lit.)
density
1.045 g/mL at 20 °C (lit.)
작용기
amine
isocyanate
phenyl
저장 온도
2-8°C
SMILES string
C[C@@H](N=C=O)c1ccccc1
InChI
1S/C9H9NO/c1-8(10-7-11)9-5-3-2-4-6-9/h2-6,8H,1H3/t8-/m1/s1
InChI key
JJSCUXAFAJEQGB-MRVPVSSYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(R)-(+)-α-Methylbenzyl isocyanate is an organic compound that belongs to the class of isocyanates. It is used in the synthesis of chiral ligands for asymmetric catalysis. These ligands play a crucial role in enantioselective reactions, allowing to produce single enantiomer products.
애플리케이션
- Paper Title: Stereoselective metabolism of nicotine and tobacco-specific N-nitrosamines to 4-hydroxy-4-(3-pyridyl)butanoic acid in rats. (Trushin N, Hecht SS, 1999).
- Paper Title: Absolute configuration of 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanol formed metabolically from 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone. (Hecht SS, Spratt TE, Trushin N, 1997).
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Inhalation - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
149.0 °F - closed cup
Flash Point (°C)
65 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Myriam Matoga et al.
Journal of enzyme inhibition and medicinal chemistry, 17(6), 375-379 (2003-04-10)
The derivatization of racemic 5-[(2-methylphenoxy)methyl]-2-amino-2-oxazoline, developed as an imidazoline binding sites ligand, with (+)-(R)-alpha-methylbenzyl isocyanate was performed in chloroform. The reaction led to two pairs of diastereomers, which were separated by RP-HPLC. A kinetic study of the derivatization reaction was
High-performance liquid chromatographic determination of the enantiomers of beta-adrenoceptor blocking agents in biological fluids. II. Studies with atenolol.
S K Chin et al.
Journal of chromatography, 489(2), 438-445 (1989-04-14)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.