모든 사진(1)
About This Item
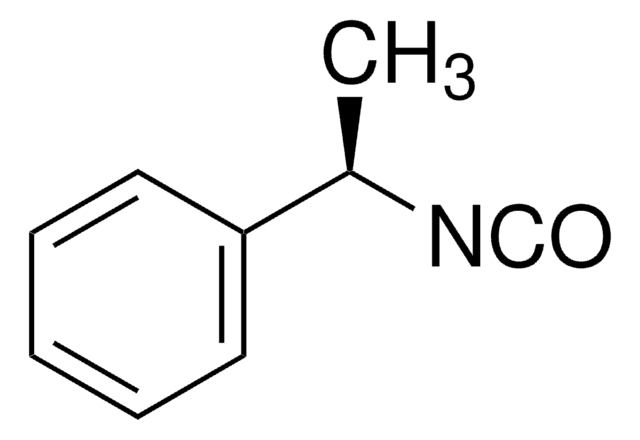
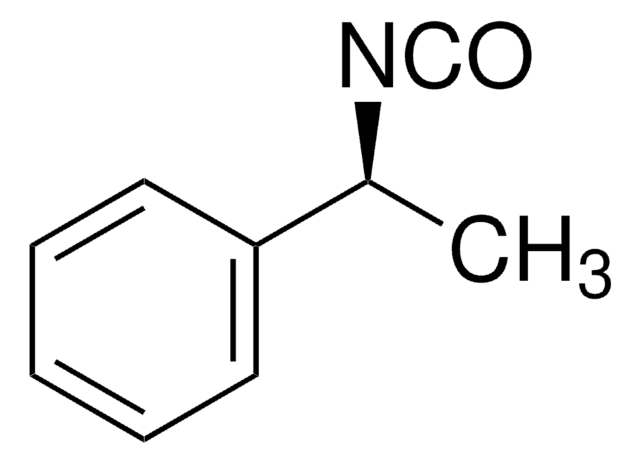
Linear Formula:
C6H5CH(CH3)NCO
CAS Number:
Molecular Weight:
147.17
Beilstein:
4230972
EC Number:
MDL number:
UNSPSC 코드:
12352118
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
98%
형태
liquid
광학 활성
[α]20/D −10°, neat
광학 순도
ee: 96% (GLC)
refractive index
n20/D 1.5145 (lit.)
bp
55-56 °C/2.5 mmHg (lit.)
density
1.045 g/mL at 20 °C (lit.)
작용기
amine
isocyanate
phenyl
저장 온도
2-8°C
SMILES string
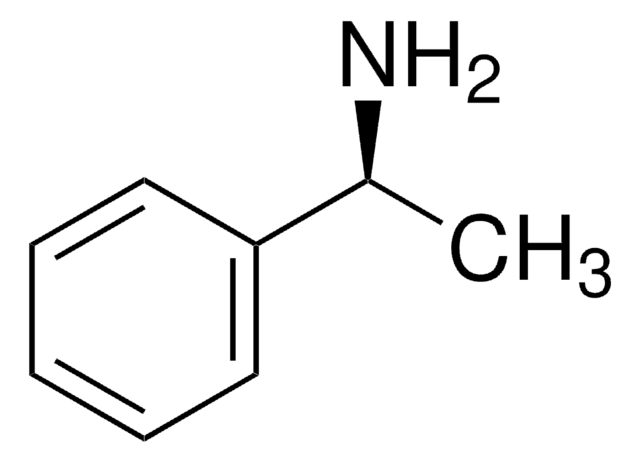
C[C@H](N=C=O)c1ccccc1
InChI
1S/C9H9NO/c1-8(10-7-11)9-5-3-2-4-6-9/h2-6,8H,1H3/t8-/m0/s1
InChI key
JJSCUXAFAJEQGB-QMMMGPOBSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
(S)-(-)-α-Methylbenzyl isocyanate is a commonly used chiral derivatizing agent for the resolution of enantiomers.
애플리케이션
(S)-(-)-α-Methylbenzyl isocyanate may be used as a chiral auxiliary for the resolution of racemic (±)-6aR,11aR-maackiain to give the (-)-form, which is a key intermediate for synthesizing (-)-cabenegrin A-I.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 2 Inhalation - Aquatic Chronic 3 - Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
149.0 °F - closed cup
Flash Point (°C)
65 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Resolution of salbutamol enantiomers in human urine by reversed-phase high performance liquid chromatography after derivatization with (S)-(-)-a-methylbenzyl isocyanate.
Kim KH, et al.
Archives of Pharmacal Research, 20(5), 486-490 (1997)
Absolute configuration and total synthesis of (-)-cabenegrin AI.
Tokes AL, et al.
Tetrahedron, 55(30), 9283-9296 (1999)
Determination of pindolol enantiomers in human plasma and urine by simple liquid-liquid extraction and high-performance liquid chromatography.
Beal JL and Tett SE
Journal of Chromatography. B, Biomedical Applications, 715(2), 409-415 (1998)
Determination of the optical purity of (R)-terbutaline by 1 H-NMR and RP-LC using chiral derivatizing agent,(S)-(-)-a-methylbenzyl isocyanate.
Kim KH, et al.
Journal of Pharmaceutical and Biomedical Analysis, 25(5), 947-956 (2001)
Myriam Matoga et al.
Journal of enzyme inhibition and medicinal chemistry, 17(6), 375-379 (2003-04-10)
The derivatization of racemic 5-[(2-methylphenoxy)methyl]-2-amino-2-oxazoline, developed as an imidazoline binding sites ligand, with (+)-(R)-alpha-methylbenzyl isocyanate was performed in chloroform. The reaction led to two pairs of diastereomers, which were separated by RP-HPLC. A kinetic study of the derivatization reaction was
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.