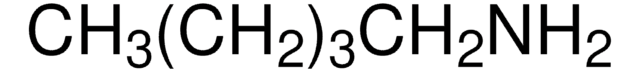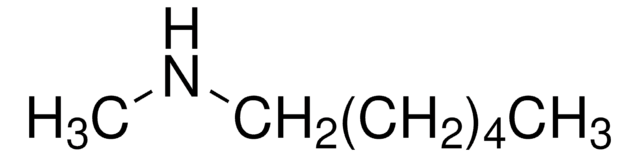추천 제품
Quality Level
분석
99%
형태
liquid
expl. lim.
2.1-9.3 %
refractive index
n20/D 1.418 (lit.)
bp
131-132 °C (lit.)
mp
−23 °C (lit.)
density
0.766 g/mL at 25 °C (lit.)
작용기
amine
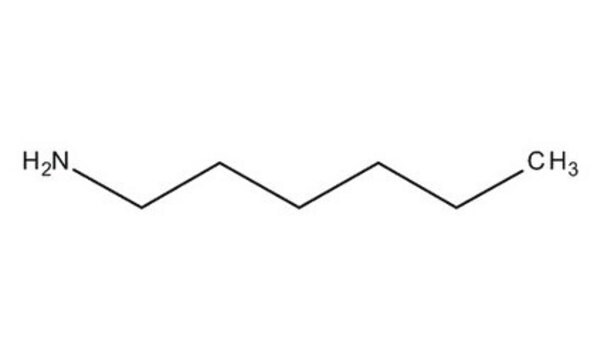
SMILES string
CCCCCCN
InChI
1S/C6H15N/c1-2-3-4-5-6-7/h2-7H2,1H3
InChI key
BMVXCPBXGZKUPN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- As an initiator to synthesize defined polypeptides by primary amine-initiated N-carboxyanhydride ring opening polymerization reaction.
- As a reactant to modify alkanethiol monolayers at polycrystalline gold surfaces via amide bond formation reaction.
- To functionalize the surface of MWCNT, graphene oxide, and polyurethanes. These functionalized composites materials find applications in absorption, CO2 capture, and as barrier materials.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Flam. Liq. 3 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
80.6 °F - closed cup
Flash Point (°C)
27 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
이미 열람한 고객
프로토콜
Information on the Amide bond and the Catalytic Amide Bond Formation Protocol. Amidation of amines and alcohols. The amide bond, an important linkage in organic chemistry, is a key functional group in peptides, polymers, and many natural products and pharmaceuticals.
Separation of Propylamine; Butylamine; Pentylamine; Hexylamine; Heptylamine; Octylamine; Nonylamine; Decylamine
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.