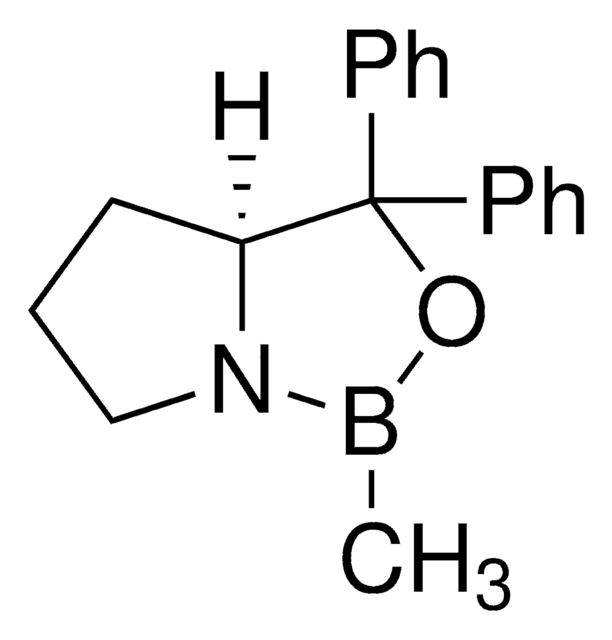
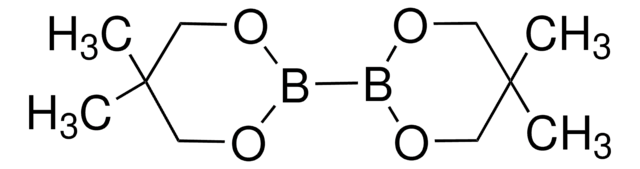
추천 제품
Quality Level
분석
98%
양식
liquid
반응 적합성
reagent type: reductant
refractive index
n20/D 1.507 (lit.)
bp
50 °C/50 mmHg (lit.)
mp
12 °C (lit.)
density
1.125 g/mL at 25 °C (lit.)
저장 온도
2-8°C
SMILES string
[bH]1oc2ccccc2o1
InChI
1S/C6H5BO2/c1-2-4-6-5(3-1)8-7-9-6/h1-4,7H
InChI key
CENMEJUYOOMFFZ-UHFFFAOYSA-N
애플리케이션
A monofunctional hydroborating agent which reduces β-hydroxyketones to 1,3-diols. Effects conjugate reduction of α,β-enones.
Used to prepare B-alkylcatecholboranes which were used, in turn, to generate alkyl radicals forming aryl ethers from quinones. Employed in a preparation of C2-symmetric boron complexes from methylenebis(oxazolines) used for enantioselective reduction of ketones.
법적 정보
Made under U.S. Pat. No. 6,204,405.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
35.6 °F - closed cup
Flash Point (°C)
2 °C - closed cup
개인 보호 장비
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
이미 열람한 고객
The Journal of Organic Chemistry, 55, 5678-5678 (1990)
The Journal of Organic Chemistry, 55, 5190-5190 (1990)
European Journal of Organic Chemistry, 4596-4596 (2006)
Eveline Kumli et al.
Organic letters, 8(25), 5861-5864 (2006-12-01)
Addition of alkyl radicals generated from B-alkylcatecholboranes onto 1,4-benzoquinones leads to substituted hydroquinones in good overall yields. Formation of aryl ethers via a unique radical addition to the oxygen atom of the enone system is the main reaction when bulky
문서
Arylboronic acids and esters are invaluable tools for the chemical community. These powerful reagents are used for a variety of transformations, most notably the Suzuki-Miyaura cross-coupling reaction.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.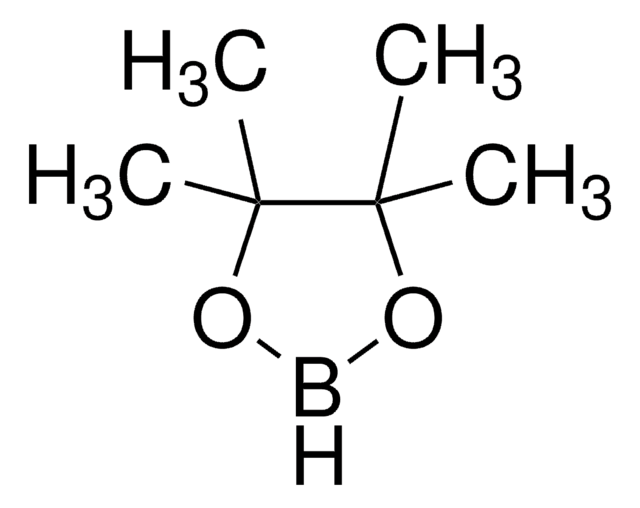
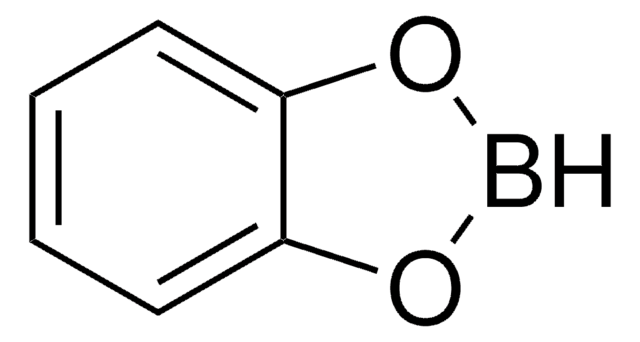
![9-Borabicyclo[3.3.1]nonane solution 0.5 M in THF](/deepweb/assets/sigmaaldrich/product/structures/180/891/8b64e597-269d-4780-98b6-40889dfd06b9/640/8b64e597-269d-4780-98b6-40889dfd06b9.png)




![9-Borabicyclo[3.3.1]nonane dimer](/deepweb/assets/sigmaaldrich/product/structures/203/431/624973a6-aec1-4b23-b6c4-013285ac418c/640/624973a6-aec1-4b23-b6c4-013285ac418c.png)