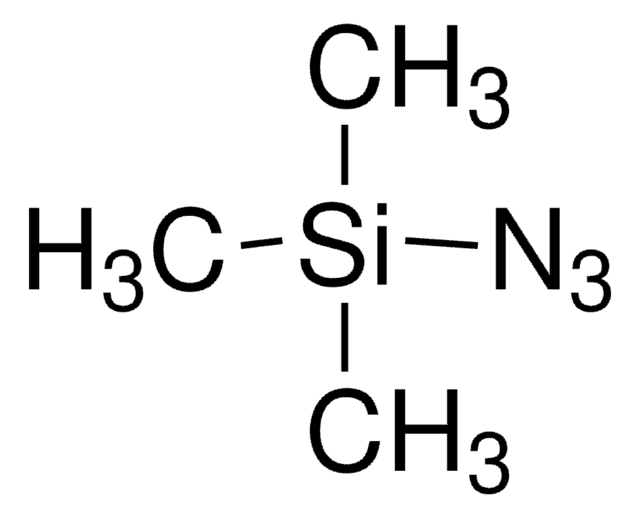추천 제품
Quality Level
분석
97%
양식
liquid
반응 적합성
reaction type: click chemistry
refractive index
n20/D 1.551 (lit.)
bp
157 °C/0.17 mmHg (lit.)
density
1.277 g/mL at 25 °C (lit.)
작용기
azide
저장 온도
2-8°C
SMILES string
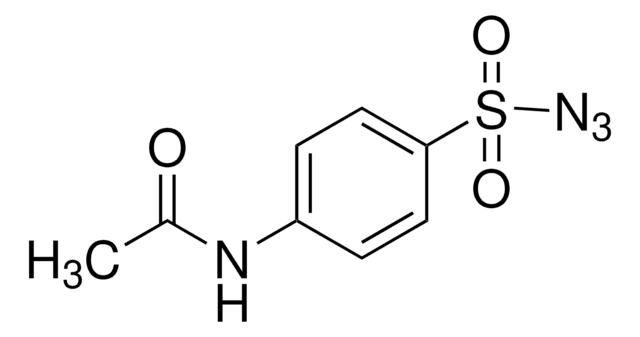
[N-]=[N+]=NP(=O)(Oc1ccccc1)Oc2ccccc2
InChI
1S/C12H10N3O3P/c13-14-15-19(16,17-11-7-3-1-4-8-11)18-12-9-5-2-6-10-12/h1-10H
InChI key
SORGEQQSQGNZFI-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Reagent for synthesis of oligosaccharides linked with carbamate and urea bonds utilizing modified Curtis rearrangement
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
233.6 °F - closed cup
Flash Point (°C)
112 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
문서
The generation of an acid chloride is an obvious way to activate the carboxy group for amide bond formation. However, practical application of acid chlorides in peptide synthesis is restricted, because they are prone to side reactions and racemization.
The chemistry of organoazides is exceedingly rich, since the azide functionality reacts with electrophiles, nucleophiles, and dipolarophiles, with or without the extrusion of dinitrogen. Common place transformation such as Staudinger reductions or ligations, Cu(I)-catalyzed Huisgen cycloadditions (of the “click” reaction family), Curtius or Schmidt rearrangents, nitrene reactions, or imine formation via aza-Wittig reactions all necessitate organoazide precursors or intermediates
Since the preparation of the first organic azide, phenyl azide, by Peter Griess in 1864 this energy-rich and versatile class of compounds has enjoyed considerable interest.
Global Trade Item Number
| SKU | GTIN |
|---|---|
| 178756-1KG | |
| 178756-25G | 4061838753939 |
| 178756-5G | 4061838753946 |
| 178756-100G | 4061838753922 |
| 178756-25KG | |
| 178756-500G | 4061838134875 |
| 178756-5KG | 4061838125224 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.
![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)


![1,4-Diazabicyclo[2.2.2]octane ReagentPlus®, ≥99%](/deepweb/assets/sigmaaldrich/product/structures/366/129/a6ff4175-974d-4fac-9038-b35e508ef252/640/a6ff4175-974d-4fac-9038-b35e508ef252.png)