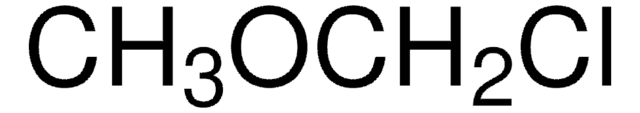142670
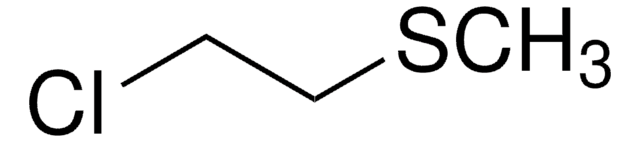
Chloromethyl ethyl ether
95%
동의어(들):
(Chloromethoxy)ethane, Ethoxychloromethane, Ethoxymethyl chloride, Ethyl chloromethyl ether
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
C2H5OCH2Cl
CAS Number:
Molecular Weight:
94.54
EC Number:
MDL number:
UNSPSC 코드:
12352101
PubChem Substance ID:
NACRES:
NA.22
추천 제품
Quality Level
분석
95%
양식
liquid
refractive index
n20/D 1.404 (lit.)
bp
82 °C (lit.)
density
1.019 g/mL at 25 °C (lit.)
작용기
chloro
ether
저장 온도
2-8°C
SMILES string
CCOCCl
InChI
1S/C3H7ClO/c1-2-5-3-4/h2-3H2,1H3
InChI key
FCYRSDMGOLYDHL-UHFFFAOYSA-N
애플리케이션
Chloromethyl ethyl ether can be used to prepare:
- 6-Benzyl-1-(ethoxymethyl)-5-iodopyrimidine-2,4(1H,3H)-dione, a key intermediate in the preparation of MKC-442 analog.
- Acylation catalyst for alcohols named 1,3-bis-[(R)-1-(2-naphthyl)ethyl]imidazoliumchloride by reacting with glyoxal-bis-[(R)-1-(2-naphthyl)ethyl]imine.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Flam. Liq. 2
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point (°F)
66.2 °F - closed cup
Flash Point (°C)
19 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
K Takahashi et al.
Mutation research, 156(3), 153-161 (1985-06-01)
The mutagenic characteristics of formaldehyde on bacteria were examined. All the tester strains of Escherichia coli deficient in DNA-repair enzymes tested in the present study were significantly more sensitive to the killing effect of formaldehyde than the corresponding wild-type strain.
Chiral N-heterocyclic carbenes as asymmetric acylation catalysts
Suzuki Y, et al.
Tetrahedron, 62(2-3), 302-310 (2006)
bis(Chloromethyl) ether and technical-grade chloromethyl methyl ether.
Report on carcinogens : carcinogen profiles, 10, 56-57 (2004-08-24)
Tadeusz J Szalaty et al.
International journal of biological macromolecules, 119, 431-437 (2018-07-29)
In this research we use ionic liquids in combination with mild process conditions to provide a selective increase in the content of carbonyl groups in the kraft lignin structure. Such modification can improve the properties of the pristine biopolymer. In
Three Routes for the Synthesis of 6-Benzyl-1-ethoxymethyl-2, 4-dioxo-1, 2, 3, 4-tetrahydropyrimidine-5-carbaldehyde
Petersen L, et al.
Synthesis, 2001(04), 0559-0564 (2001)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.