詳細
We are committed to bringing you greener alternative products, which adhere to one or more of The 12 Principles of Green Chemistry. This antibody is Preservative-free, produced without the harm or sacrifice of animals and exceptionally stable to allow for ambient shipping and storage if needed and thus aligns with "Waste Prevention", "Designing Safer Chemicals" and "Design for Energy Efficiency".
Click here for more information.
ZooMAb® antibodies represent an entirely new generation of recombinant monoclonal antibodies. Each ZooMAb® antibody is manufactured using our proprietary recombinant expression system, purified to homogeneity, and precisely dispensed to produce robust and highly reproducible lot-to-lot consistency. Only top-performing clones are released for use by researchers. Each antibody is validated for high specificity and affinity across multiple applications, including its most commonly used application. ZooMAb® antibodies are reliably available and ready to ship when you need them.
特異性
Clone 9-4-3 is a ZooMAb® mouse recombinant monoclonal antibody that specifically detects Fusion glycoprotein F0 in human parainfluenza 3 virus.
免疫原
Recombinant Fusion (F) glycoprotein of human parainfluenza virus type 3.
アプリケーション
Quality Control Testing
Evaluated by ELISA with recombinant Fusion Glycoprotein F0 of Human parainfluenza virus type 3.
ELISA Analysis: A serial of dilutions of this antibody detected recombinant Fusion Glycoprotein F0 of Human parainfluenza virus type 3.
Tested Applications
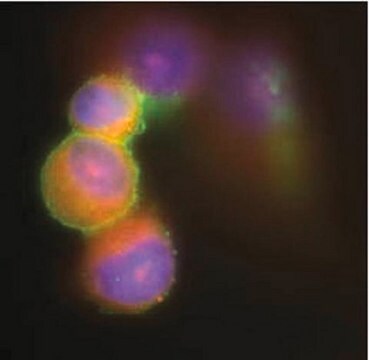
Immunocytochemistry Analysis: A 1:100 dilution from a representative lot detected Fusion Glycoprotein F0 in HPIV-3 infected LLC-MK2 cells, but not in and uninfected cells.
Affinity Binding Assay: A representative lot of this antibody bound recombinant Fusion Glycoprotein F0 of Human parainfluenza virus type 3 with a KD of 2.3 x 10-7 in an affinity binding assay.
Note: Actual optimal working dilutions must be determined by end user as specimens, and experimental conditions may vary with the end user.
ターゲットの説明
Fusion glycoprotein F0 (UniProt: P06828; also known as F0) is encoded by the F gene (Gene ID: 911957) in human parainfluenza 3 virus. The envelope of human parainfluenza virus type 3 (HPF3) contains two viral glycoproteins, the hemagglutinin-neuraminidase (HN) and the fusion protein (F). HN possesses neuraminidase activity and can cleave the sialic acid moiety of the sialic acid-containing cellular receptors on cell surfaces and promote the release of newly formed virions from the cell surface, thus allowing these virions to penetrate additional cells. Fusion glycoprotein F0 is a single-pass type I membrane glycoprotein that is synthesized with a signal peptide (aa 1-18), which is subsequently cleaved off to produce the mature, but inactive precursor protein that contains an extracellular domain (aa 19-493), a transmembrane domain (aa 494-514), and a cytoplasmic domain (aa 515-539). Following its synthesis, the inactive F0 is glycosylated and proteolytically cleaved into fusion glycoprotein 1 (aa 110-539) and fusion glycoprotein F2 (aa 19-109) that are functionally active. The cleavage is mediated by cellular proteases during the transport and maturation of the polypeptide. F0 is considered as a class I viral fusion protein that is present in at least three conformational states: a pre-fusion native state, pre-hairpin intermediate state, and post-fusion hairpin state. During viral and plasma cell membrane fusion, the heptad repeat (HR) regions assume a trimer-of-hairpins structure, positioning the fusion peptide in close proximity to the C-terminal region of the ectodomain. The formation of this structure drives apposition and subsequent fusion of viral and plasma cell membranes. This fusion is pH independent and occurs directly at the outer cell membrane. The N- terminal region of F1 is shown to be highly hydrophobic and is thought to make the first contact with the lipid membrane during virus-cell fusion. This ZooMAb® recombinant monoclonal antibody, generated by our propriety technology, offers significantly enhanced specificity, affinity, reproducibility, and stability over conventional monoclonals. (Ref.: Hendrickson, KJ. (2003). Clin. Microbiol. Rev. 16(2); 242-264; Porotto, M., et al. (2001). J Virol. 75(16); 7481-7488).
物理的形状
Purified recombinant mouse monoclonal antibody IgG, lyophilized in PBS, 5% Trehalose, normal appearance a coarse or translucent resin. The PBS/trehalose components in the ZooMAb formulation can have the appearance of a semi-solid (bead like gel) after lyophilization. This is a normal phenomenon. Please follow the recommended reconstitution procedure in the data sheet to dissolve the semi-solid, bead-like, gel-appearing material. The resulting antibody solution is completely stable and functional as proven by full functional testing. Contains no biocide or preservatives, such as azide, or any animal by-products. Larger pack sizes provided as multiples of 25 µL.
再構成
0.3 mg/mL after reconstitution at 25 µL per vial. Please refer to guidance on suggested starting dilutions and/or titers per application and sample type.
保管および安定性
Recommend storage of lyophilized product at 2-8°C; Before reconstitution, micro-centrifuge vials briefly to spin down material to bottom of the vial; Reconstitute each vial by adding 25 µL of filtered lab grade water or PBS; Reconstituted antibodies can be stored at 2-8°C, or -20°C for long term storage. Avoid repeated freeze-thaws.
その他情報
Concentration: Please refer to the Certificate of Analysis for the lot-specific concentration.
法的情報
ZooMAb is a registered trademark of Merck KGaA, Darmstadt, Germany
免責事項
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.