おすすめの製品
由来生物
rabbit
品質水準
結合体
unconjugated
抗体製品の状態
IgG fraction of antiserum
抗体製品タイプ
primary antibodies
クローン
polyclonal
フォーム
buffered aqueous solution
分子量
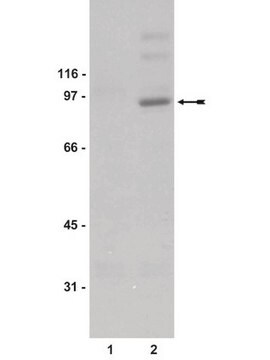
46 kDa
交差性
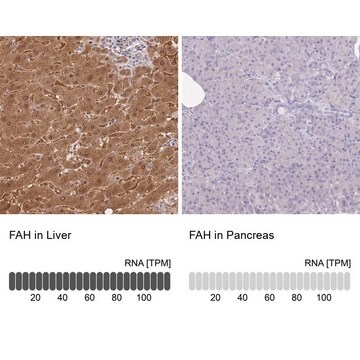
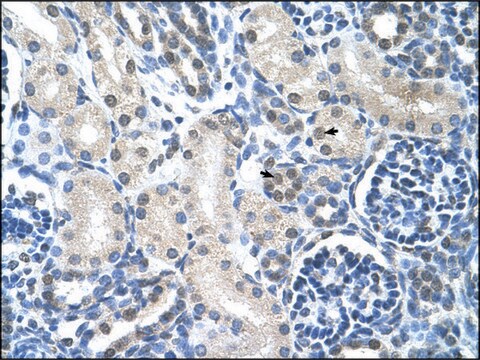
dog, horse, human, mouse, rat
濃度
0.5-1 mg/mL
テクニック
immunoblotting: suitable
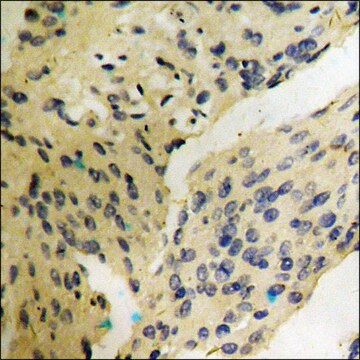
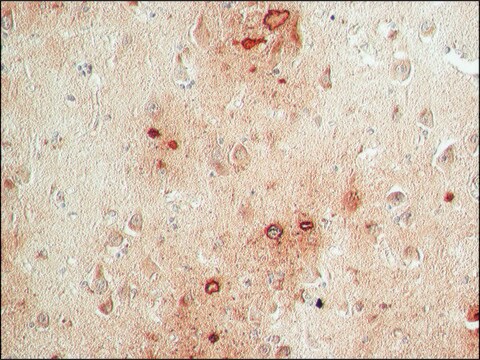
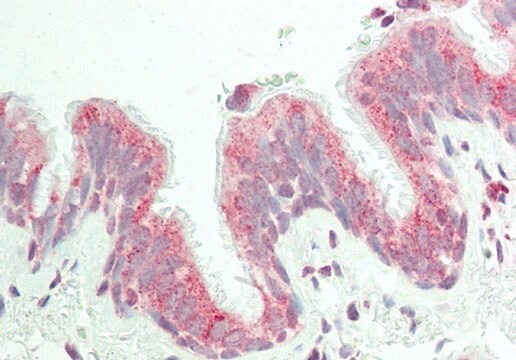
immunohistochemistry: suitable
アクセッション番号
NM_000137
UniProtアクセッション番号
輸送温度
wet ice
保管温度
−20°C
ターゲットの翻訳後修飾
unmodified
遺伝子情報
human ... FAH(2184)
詳細
The FAH gene is mapped to human chromosome 15q25.1. The encoded protein consists of 419 amino acids and 14 coding exons.
免疫原
Synthetic peptide directed towards the C terminal region of human FAH
生物化学的/生理学的作用
FAH (fumarylacetoacetate hydrolase) catalyzes the last step in tyrosine catabolic pathway. FAH deficiency leads to hereditary tyrosinemia type 1. FAH mutations eventually lead to Renal Fanconi Syndrome, due to severe liver cirrhosis and renal tubular acidosis caused by accumulation of toxic metabolites such as fumarylacetoacetate.
FAH is the last enzyme in the tyrosine catabolism pathway. FAH deficiency is associated with Type 1 hereditary tyrosinemia.This gene encodes the last enzyme in the tyrosine catabolism pathway. FAH deficiency is associated with Type 1 hereditary tyrosinemia (HT).
シーケンス
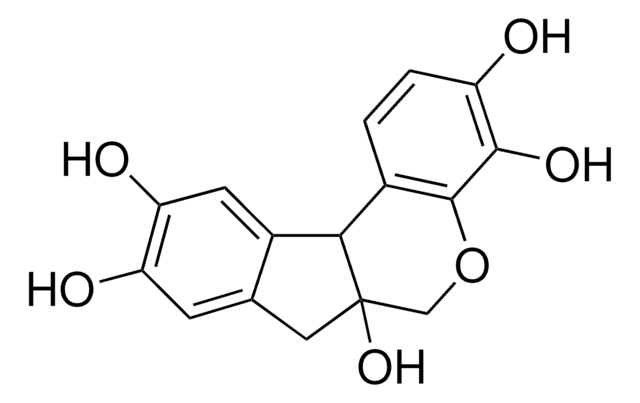
Synthetic peptide located within the following region: AATICKSNFKYMYWTMLQQLTHHSVNGCNLRPGDLLASGTISGPEPENFG
物理的形状
Purified antibody supplied in 1x PBS buffer with 0.09% (w/v) sodium azide and 2% sucrose.
免責事項
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
保管分類コード
10 - Combustible liquids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
SAB2108553-100UL:
最新バージョンのいずれかを選択してください:
Molecular Aspects of the FAH Mutations Involved in HT1 Disease.
Morrow G, et al.
Advances in Experimental Medicine and Biology, 25-48 (2017)
Silent tyrosinemia type I without elevated tyrosine or succinylacetone associated with liver cirrhosis and hepatocellular carcinoma.
Blackburn P R, et al.
Human Mutation, 37(10), 1097-1105 (2016)
Biochemical and molecular diagnosis of tyrosinemia type I with two novel FAH mutations in a Hong Kong chinese patient: Recommendation for expanded newborn screening in Hong Kong
Mak CM, et al.
Clinical Biochemistry, 46(1-2), 155-159 (2013)
Marco De Giorgi et al.
Molecular therapy. Methods & clinical development, 21, 656-669 (2021-06-19)
Clinical application of somatic genome editing requires therapeutics that are generalizable to a broad range of patients. Targeted insertion of promoterless transgenes can ensure that edits are permanent and broadly applicable while minimizing risks of off-target integration. In the liver
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)