おすすめの製品
product name
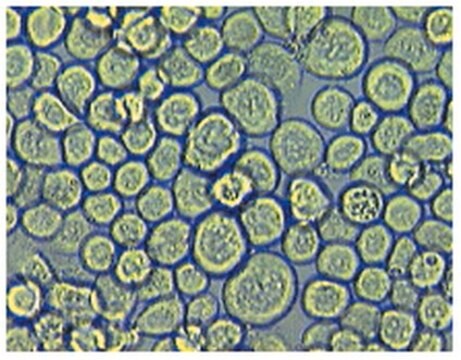
Frozen Human Systemic Lupus Erythematosus (SLE) PBMC, 50 Million Cells/per vial
由来生物
human peripheral blood (mononuclear cells)
品質水準
形状
frozen liquid
包装
vial of 50 Million cells
メーカー/製品名
BioIVT
テクニック
cell culture | mammalian: suitable
色
cloudy
保管温度
-140 to -196°C
詳細
Lot specific orders are not able to be placed through the web. Contact your local sales rep for more details.
We are pleased to offer these products from BioIVT.
Offering isolated Peripheral Blood Mononuclear Cells (PBMCs) from whole blood procured from IRB-consented donors with clinically defined Systemic Lupus Erythematosus (SLE).
Peripheral Blood Mononuclear Cells (PBMCs) are a subset of leukocytes (or White Blood Cell, WBC), which are integral components of the body′s immune system that fight disease.
PBMCs consist of a mixture of lymphocytes (T cells, B cells, and NK cells) and monocytes and are isolated from peripheral whole blood via density gradient centrifugation and red blood cell lysis.
Standard and custom clinical data collection and confirmation ensures proper corresponding disease severity and treatment history.
Clinical data may include: Medication (current and historical), Disease severity, Standard blood chemistry results, dsDNA results, C3/C4 results, ANA results
PBMCs are provided with clinical data that has been collected following a strict and standardized clinical data process. All specimens are reviewed for IRB approval.
We are pleased to offer these products from BioIVT.
Offering isolated Peripheral Blood Mononuclear Cells (PBMCs) from whole blood procured from IRB-consented donors with clinically defined Systemic Lupus Erythematosus (SLE).
Peripheral Blood Mononuclear Cells (PBMCs) are a subset of leukocytes (or White Blood Cell, WBC), which are integral components of the body′s immune system that fight disease.
PBMCs consist of a mixture of lymphocytes (T cells, B cells, and NK cells) and monocytes and are isolated from peripheral whole blood via density gradient centrifugation and red blood cell lysis.
Standard and custom clinical data collection and confirmation ensures proper corresponding disease severity and treatment history.
Clinical data may include: Medication (current and historical), Disease severity, Standard blood chemistry results, dsDNA results, C3/C4 results, ANA results
PBMCs are provided with clinical data that has been collected following a strict and standardized clinical data process. All specimens are reviewed for IRB approval.
包装
50 Million Cells/per vial
その他情報
Thaw before use.
免責事項
RESEARCH USE ONLY. This product is regulated in France when intended to be used for scientific purposes, including for import and export activities (Article L 1211-1 paragraph 2 of the Public Health Code). The purchaser (i.e. enduser) is required to obtain an import authorization from the France Ministry of Research referred in the Article L1245-5-1 II. of Public Health Code. By ordering this product, you are confirming that you have obtained the proper import authorization.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Met. Corr. 1 - Skin Irrit. 2
保管分類コード
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
労働安全衛生法名称等を表示すべき危険物及び有害物
名称等を表示すべき危険物及び有害物
労働安全衛生法名称等を通知すべき危険物及び有害物
名称等を通知すべき危険物及び有害物
Jan Code
HUMANPBMC-0002181:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)