おすすめの製品
品質水準
アッセイ
≥98%
mp
200-201 °C (lit.)
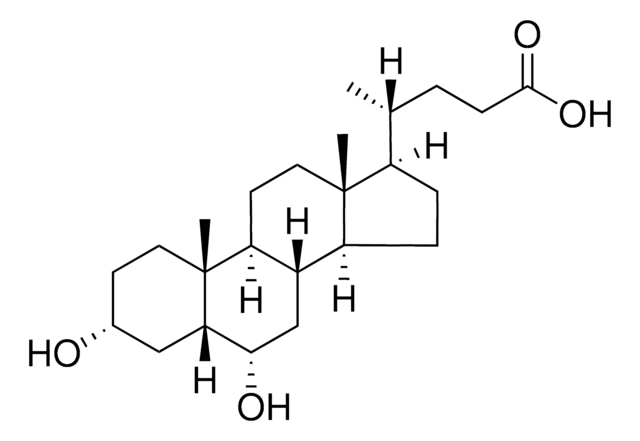
SMILES記法
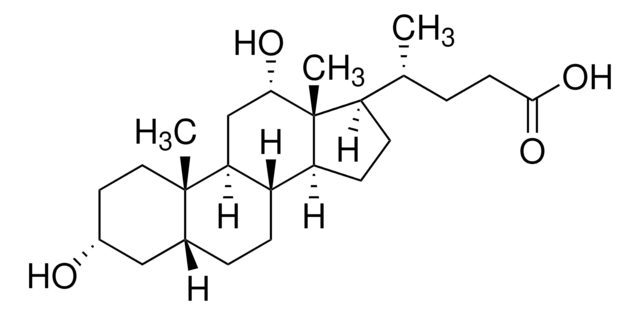
C[C@H](CCC(O)=O)[C@H]1CC[C@H]2[C@@H]3C[C@H](O)[C@@H]4C[C@H](O)CC[C@]4(C)[C@H]3CC[C@]12C
InChI
1S/C24H40O4/c1-14(4-7-22(27)28)17-5-6-18-16-13-21(26)20-12-15(25)8-10-24(20,3)19(16)9-11-23(17,18)2/h14-21,25-26H,4-13H2,1-3H3,(H,27,28)/t14-,15-,16+,17-,18+,19+,20+,21+,23-,24-/m1/s1
InChI Key
DGABKXLVXPYZII-SIBKNCMHSA-N
遺伝子情報
human ... NR1H2(7376) , NR1H3(10062)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
アプリケーション
- as a standard for serum bile acid profiling by liquid chromatography-mass spectrometry (LC-MS) method in rat cells.
- as a component of the growth medium to study its effect as an anti-aging small molecule on the chronological life span (CLS) of the pex5Δ (Δ) strain.
- as a standard for analyzing bile acid composition and total bile acids in mice cells.
よく一緒に購入される製品
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
H3878-5G:
H3878-25G:
H3878-VAR:
H3878-BULK:
H3878-1G:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)