おすすめの製品
詳細
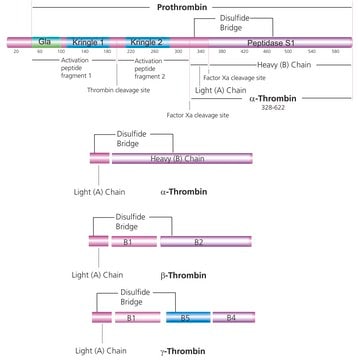
Anti Factor IX antibody, Mouse monoclonal (mouse IgG1 isotype) is derived from the HIX-1 hybridoma produced by the fusion of mouse Sp2/0-Ag14 myeloma cells and splenocytes from BALB/c mice immunized with factor IX purified from human plasma. Factor IX concentration in human plasma ranges between 2.5-5 mg/ml and its half-life is approximately 24 hours. The human factor IX gene is about 40 Kb in size and is localized at the distal end of the X-chromosome.
特異性
第IX因子の精製や第IX因子を枯渇させたヒト血漿の調製に利用することができます。
免疫原
プ-ルされた正常なヒト血漿由来の第Ⅸ因子です。
アプリケーション
Applications in which this antibody has been used successfully, and the associated peer-reviewed papers, are given below.
Enzyme-linked immunosorbent assay (1 paper)
Enzyme-linked immunosorbent assay (1 paper)
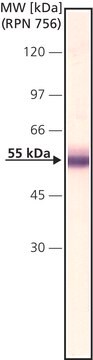
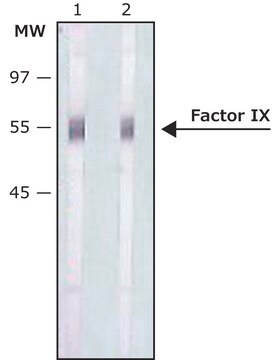
Monoclonal Anti-Factor IX antibody produced in mouse is suitable for ELISA-based assay in detection of FIX Inhibitors and it has been used as a positive control in ELISA. It is also suitable for western blot at a concentration of 2-4μg/mL.
生物化学的/生理学的作用
FIX plays a key role in the intrinsic and extrinsic blood coagulation systems. It is synthesized in liver and later upon activation in plasma it gets converted into a serine protease. In second step, FIX undergoes series of complex post-translation modifications such as γ-carboxylation of 12 N-terminal glutamic acid residues, N- and O-linked glycosylation, phosphorylation, β-hydroxylation, sulfation, disulfide bond formation etc. It is secreted into the plasma after completion of post-translation modifications. Hereditary deficiencies or dysfunctions of factor IX cause hemophilia B or "Christmas Disease" (the surname of the first family described). In haemophilia B, FIX showed impaired coagulation and increased tendency to bleed as a reason of mutation in the X chromosome linked FIX gene. Factor IX is synthesized in liver parenchymal cells and requires a post-translational vitamin K-dependent modification in order to become a mature plasma zymogen. When patients lack vitamin-K or take oral anticoagulants that interfere with the metabolism of vitamin-K, a hypocoagulable or antithrombotic state is induced.
物理的形状
140 mM 塩化ナトリウム、0.05% アジ化ナトリウム含有のpH 7.4の10 mM HEPES溶液です。
免責事項
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
適切な製品が見つかりませんか。
製品選択ツール.をお試しください
保管分類コード
12 - Non Combustible Liquids
WGK
nwg
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
F2645-BULK:
F2645-.5ML:
F2645-.2ML:
F2645-VAR:
Jianming Liu et al.
The protein journal, 33(2), 174-183 (2014-02-26)
Recombinant human FIX concentrates (rhFIX) are essential in the treatment and prevention of bleeding in the bleeding disorder haemophilia B. However, due to the complex nature of FIX production yields are low which leads to high treatment costs. Here we
Jiamiao Lu et al.
Molecular therapy : the journal of the American Society of Gene Therapy, 25(5), 1187-1198 (2017-04-04)
Conventional plasmid vectors are incapable of achieving sustained levels of transgene expression in vivo even in quiescent mammalian tissues because the transgene expression cassette is silenced. Transcriptional silencing results from the presence of the bacterial plasmid backbone or virtually any DNA
Genetic analysis of a hemophilia B family with a novel F9 gene mutation: A STROBE-compliant article
Lv X, et al.
Medicine, 98(21), e15688-e15688 (2019)
Patricia Favaro et al.
Human gene therapy, 22(7), 843-852 (2010-12-04)
Intravascular delivery of adeno-associated virus (AAV) vector is commonly used for liver-directed gene therapy. In humans, the high prevalence of neutralizing antibodies to AAV-2 capsid and the wide cross-reactivity with other serotypes hamper vector transduction efficacy. Moreover, the safety of
S V Kovnir et al.
Acta naturae, 10(1), 51-65 (2018-05-02)
Hemophilia B patients suffer from an inherited blood-clotting defect and require regular administration of blood-clotting factor IX replacement therapy. Recombinant human factor IX produced in cultured CHO cells is nearly identical to natural, plasma-derived factor IX and is widely used
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)