おすすめの製品
品質水準
アッセイ
≥99%
フォーム
solid
SMILES記法
[H]N1C(Cl)=CC(=O)NC1=O
InChI
1S/C4H3ClN2O2/c5-2-1-3(8)7-4(9)6-2/h1H,(H2,6,7,8,9)
InChI Key
PKUFNWPSFCOSLU-UHFFFAOYSA-N
アプリケーション
Chlorouracil (4-Chlorouracil; 6-Chlorouracil) is a halogenated uracil that is useful in studies of the effects of halogenation on nucleic acid base-pair stability and alkali metal ion affinity.
Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaines are cross-conjugated and thus possess separate cationic (pyridinium) and anionic (uracilate) moieties. Calculations and X-ray single crystal analyses may be used to characterize these systems and to compare the salts with the betaines.
Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaines are cross-conjugated and thus possess separate cationic (pyridinium) and anionic (uracilate) moieties. Calculations and X-ray single crystal analyses may be used to characterize these systems and to compare the salts with the betaines.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
C9525-VAR:
C9525-10G:
C9525-BULK:
C9525-1G:
C9525-5G:
Andreas Schmidt et al.
Organic & biomolecular chemistry, 4(16), 3056-3066 (2006-08-04)
Reaction of 6-chlorouracil with 4-(dimethylamino)pyridine, 4-methylpyridine, and pyridin-4-yl-morpholine yielded pyridinium-substituted uracils as chlorides which were converted into pyridinium uracilates by deprotonation. These heterocyclic mesomeric betaines are cross-conjugated and thus possess separate cationic (pyridinium) and anionic (uracilate) moieties. Calculations and X-ray
Zhibo Yang et al.
Journal of the American Chemical Society, 126(49), 16217-16226 (2004-12-09)
The influence of halogenation on the properties of uracil and its noncovalent interactions with alkali metal ions is investigated both experimentally and theoretically. Bond dissociation energies of alkali metal ion-halouracil complexes, M+(XU), are determined using threshold collision-induced dissociation techniques in
Photochemical transformation of 6-chlorouracil and some alkylated analogues.
Z Kazimierczuk et al.
Biochimica et biophysica acta, 254(2), 157-166 (1971-12-16)
Francesca Bartoccini et al.
Organic & biomolecular chemistry, 10(44), 8860-8867 (2012-10-11)
A small library of 8-substituted 9-deazaxanthines has been prepared by late-stage diversification of an 8-bromo-9-deazaxanthine. By utilizing palladium-catalyzed cross-coupling reactions a single key precursor can be transformed into a variety of 8-substituted-9-deazaxanthine compounds. Three key 8-bromo-9-deazaxanthine intermediates were efficiently prepared
Tumor uptake of radiolabelled pyrimidine bases and pyrimidine nucleosides in animal models--V. 6-[36Cl]chlorouracil, 6-[82Br]bromouracil and 6-[123I]iodouracil.
Y W Lee et al.
International journal of nuclear medicine and biology, 11(3-4), 262-266 (1984-01-01)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)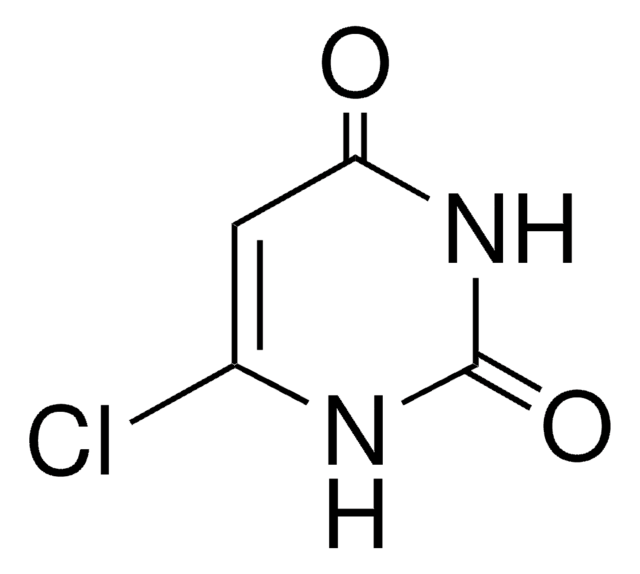


![6-Chloroimidazo[1,2-b]pyridazine hydrochloride AldrichCPR](/deepweb/assets/sigmaaldrich/product/structures/318/484/01bb1292-cad3-4f2f-86cf-0721eb3116d7/640/01bb1292-cad3-4f2f-86cf-0721eb3116d7.png)
