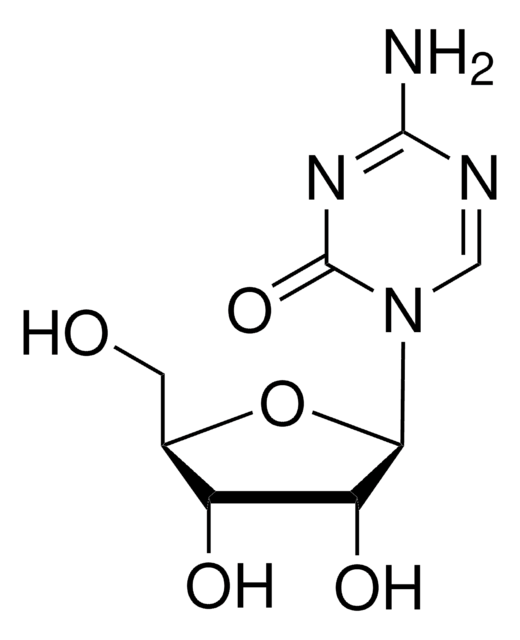
おすすめの製品
アッセイ
≥98% (HPLC)
形状
powder
色
white
溶解性
DMSO: >10 mg/mL
オーガナイザー
Sanofi Aventis
保管温度
2-8°C
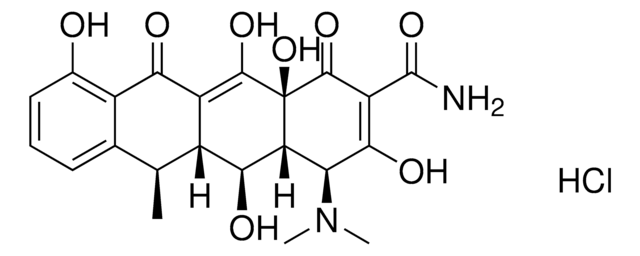
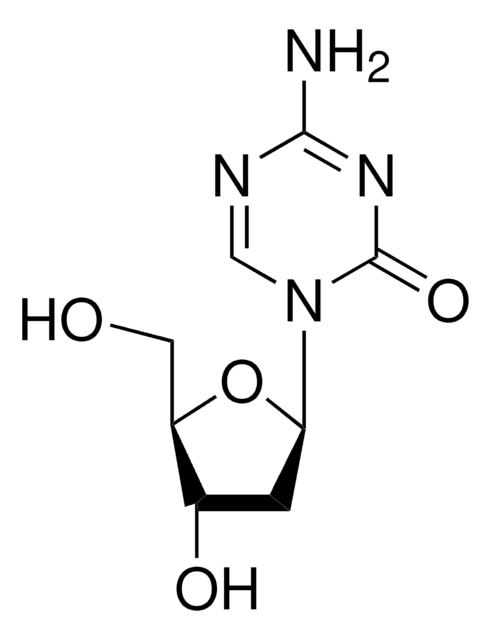
SMILES記法
Nc1nc(Cl)nc2n(cnc12)[C@@H]3O[C@H](CO)[C@@H](O)[C@@H]3F
InChI
1S/C10H11ClFN5O3/c11-10-15-7(13)5-8(16-10)17(2-14-5)9-4(12)6(19)3(1-18)20-9/h2-4,6,9,18-19H,1H2,(H2,13,15,16)/t3-,4+,6-,9-/m1/s1
InChI Key
WDDPHFBMKLOVOX-AYQXTPAHSA-N
遺伝子情報
human ... POLA1(5422) , POLA2(23649) , POLD1(5424) , POLD2(5425) , POLD3(10714) , POLD4(57804) , POLE(5426) , POLE2(5427) , POLE3(54107) , PRIM1(5557) , PRIM2(5558) , RRM1(6240) , RRM2(6241) , RRM2B(50484)
アプリケーション
生物化学的/生理学的作用
特徴および利点
シグナルワード
Warning
危険有害性の分類
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Carc. 2 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
C7495-VAR:
C7495-5MG:
C7495-BULK:
C7495-25MG:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)