おすすめの製品
アッセイ
≥90.0% (GC)
フォーム
liquid
光学活性
[α]/D -20±3°, c = 1 in ethanol
保管温度
2-8°C
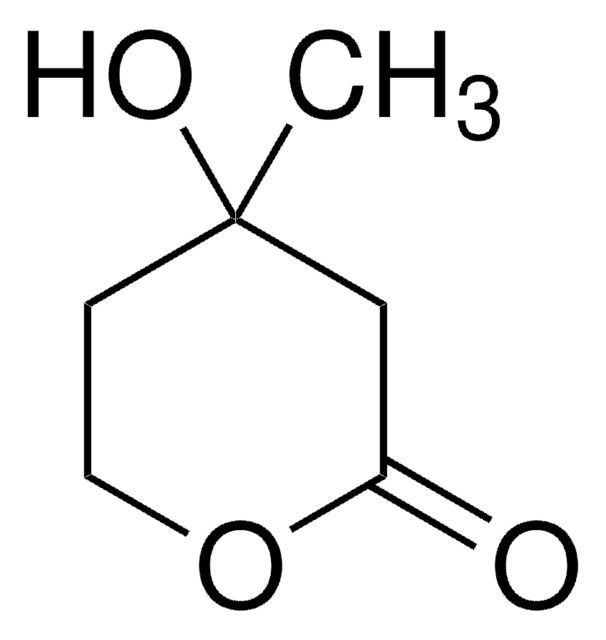
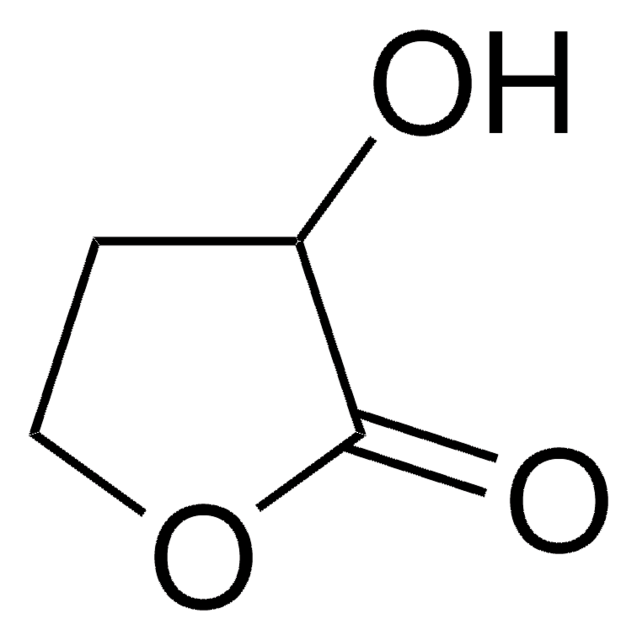
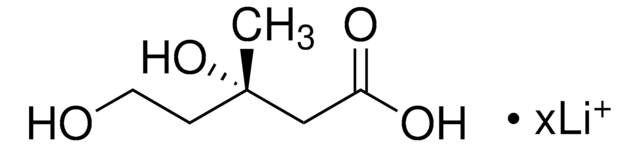
SMILES記法
C[C@@]1(O)CCOC(=O)C1
InChI
1S/C6H10O3/c1-6(8)2-3-9-5(7)4-6/h8H,2-4H2,1H3/t6-/m1/s1
InChI Key
JYVXNLLUYHCIIH-ZCFIWIBFSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
生物化学的/生理学的作用
ステロール、テルペン、カロテノイド、およびその他の天然物が得られる生合成経路において、古典的で鏡像異性的に純粋な代謝物。
包装
琥珀色の丸ボトル
保管分類コード
10 - Combustible liquids
WGK
WGK 3
引火点(°F)
235.4 °F - closed cup
引火点(℃)
113 °C - closed cup
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第三石油類
危険等級III
非水溶性液体
Jan Code
68519-VAR:
68519-500MG-BULK:
68519-100MG:
68519-500MG:
68519-100MG-BULK:
68519-BULK:
この製品を見ている人はこちらもチェック
Akira Honda et al.
Journal of lipid research, 48(5), 1212-1220 (2007-02-03)
We have developed a new sensitive and specific nonradioisotope assay method to measure the activity of HMG-CoA reductase, the rate-controlling enzyme in the cholesterol biosynthetic pathway. This method was based upon a stable isotope dilution technique by liquid chromatography-tandem mass
Jenna Waldron et al.
Annals of clinical biochemistry, 48(Pt 3), 223-232 (2011-03-01)
Mevalonic acid (MVA) is synthesized at an early and rate-limiting step in the biosynthesis of cholesterol by the enzyme hydroxymethylglutaryl coenzyme A (HMG-CoA) reductase, and is a useful measure of statin efficacy or treatment. A liquid chromatography-tandem mass spectrometry (LC-MS/MS)
Synthesis and HMG-CoA reductase inhibition of 2-cyclopropyl-4-thiophenyl-quinoline mevalonolactones.
Shikui Zhao et al.
Bioorganic & medicinal chemistry, 17(23), 7915-7923 (2009-11-03)
A novel series of 2-cyclopropyl-4-thiophenyl quinoline-based mevalonolactones were synthesized from the substituted anilines by several reactions. Among them, (4R,6S)-6-[(E)-2-(2-cyclopropyl-6-fluoro-4-(4-fluoro-thiophenyl)-quinoline-3-yl)-ethenyl]-3,4,5,6-tetrahydro-4-hydroxy-2H-pyran-2-one (1d), (4R,6S)-6-[(E)-2-(2-cyclopropyl-6-fluoro-4-(3-methoxy-thiophenyl)-quinoline-3-yl)-ethenyl]-3,4,5,6-tetrahydro-4-hydroxy-2H-pyran-2-one (1f) and (4R,6S)-6-[(E)-2-(2-cyclopropyl-6-fluoro-4,7-di(3-methoxy-thiophenyl)-quinoline-3-yl)-ethenyl]-3,4,5,6-tetrahydro-4-hydroxy-2H-pyran-2-one (1q) showed potent HMG-CoA reductase inhibitory activity comparable with pitavastatin.
Jessica Fuhrmeister et al.
Toxicology letters, 215(3), 219-227 (2012-10-25)
Statins are the most widely used drugs for the treatment of hypercholesterolemia. In spite of their overall favorable safety profile, they do possess serious myotoxic potential, whose molecular origin has remained equivocal. Here, we demonstrate in cultivated myoblasts and skeletal
Ya Sh Schwartz et al.
Bulletin of experimental biology and medicine, 148(3), 406-409 (2010-04-17)
We studied the effects of cholesterol, its oxidized derivatives mevalonate, and nuclear receptor agonists LXR, RXR, and FXR on the production of transforming growth factor-beta1 (TGF- beta1) by macrophages. After recruiting of macrophage monocytes into the focus of inflammation, the
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)