おすすめの製品
由来生物
synthetic
グレード
pharmaceutical primary standard
認証
EP
APIファミリー
ciclesonide
フォーム
powder
包装
pkg of 10 mg
メーカー/製品名
EDQM
保管条件
protect from light
溶解性
water: <0.1 g/L
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
輸送温度
ambient
保管温度
2-8°C
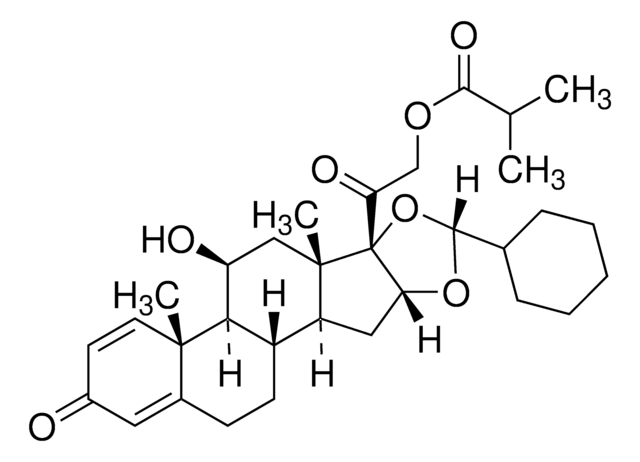
SMILES記法
O1[C@@]2([C@H](O[C@H]1C6CCCCC6)C[C@@H]3[C@@]2(C[C@@H]([C@H]4[C@H]3CCC5=CC(=O)C=C[C@@]54C)O)C)C(=O)CO
InChI
1S/C28H38O6/c1-26-11-10-18(30)12-17(26)8-9-19-20-13-23-28(22(32)15-29,27(20,2)14-21(31)24(19)26)34-25(33-23)16-6-4-3-5-7-16/h10-12,16,19-21,23-25,29,31H,3-9,13-15H2,1-2H3/t19-,20-,21-,23+,24+,25+,26-,27-,28+/m0/s1
InChI Key
OXPLANUPKBHPMS-ZXBNPROVSA-N
詳細
Ciclesonide impurity B is an impurity of ciclesonide, a new-generation, non-halogenated glucocorticoid.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets, have been developed and issued under the Authority of the issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Ciclesonide impurity B is used as an EP reference standard to quantify the analyte in pharmaceutical formulations by liquid chromatography (LC) technique.
包装
この製品は発行元の薬局方による供給としてお届けします。現在の単位量については、EDQM reference substance catalogueをご覧ください。
その他情報
Sales restrictions may apply.
最新バージョンのいずれかを選択してください:
New Drugs for Asthma, Allergy and COPD, 42, 42-54 (2001)
Rethinking Cleaning Validation for API Manufacturing
Zhang C, et al.
Pharmaceutical Technology, 42, 42-54 (2018)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)