おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
iotrolan
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
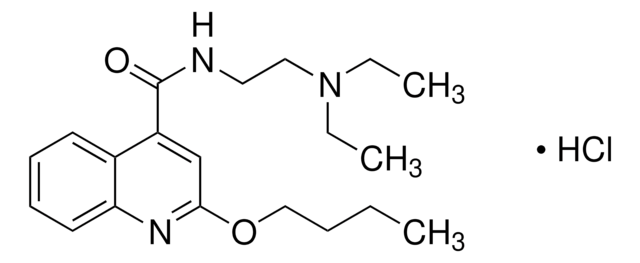
SMILES記法
Ic1c(c(c(c(c1C(=O)NC(C(O)CO)CO)I)C(=O)NC(C(O)CO)CO)I)N(C)C(=O)CC(=O)N(C)c2c(c(c(c(c2I)C(=O)NC(C(O)CO)CO)I)C(=O)NC(C(O)CO)CO)I
InChI
1S/C37H48I6N6O18/c1-48(32-28(40)22(34(64)44-12(4-50)16(58)8-54)26(38)23(29(32)41)35(65)45-13(5-51)17(59)9-55)20(62)3-21(63)49(2)33-30(42)24(36(66)46-14(6-52)18(60)10-56)27(39)25(31(33)43)37(67)47-15(7-53)19(61)11-57/h12-19,50-61H,3-11H2,1-2H3,(H,44,64)(H,45,65)(H,46,66)(H,47,67)
InChI Key
XUHXFSYUBXNTHU-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
アプリケーション
Iotrolan for system suitability EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
その他情報
Sales restrictions may apply.
最新バージョンのいずれかを選択してください:
[Prospects of the use of non-ionic X-ray contrast substances during endoscopic retrograde cholangiopancreatography].
N K Sviridov et al.
Vestnik rentgenologii i radiologii, (6)(6), 62-63 (2003-12-17)
W Krause et al.
Investigative radiology, 37(12), 698-705 (2002-11-26)
Use of near-infrared reflection spectroscopy (NIR-RS) as a new model to assess renal tolerance of contrast agents and determination of the effects of a prostacyclin analogue and of two phosphodiesterase inhibitors on renal tolerance. NIR-RS was used to measure total
Jolanda J de Poorter et al.
The journal of gene medicine, 7(11), 1421-1428 (2005-06-25)
Loosening is a major complication in prosthesis surgery. To stabilize loosened orthopedic implants, the interface tissue surrounding the implant must be removed. As an alternative to manual removal, we explored the possibility of removing the tissue by gene-directed enzyme prodrug
Ingrid Böhm et al.
European journal of radiology, 55(3), 431-436 (2005-09-01)
To test whether mono- or dimeric X-ray contrast media (CM) may induce the de novo production of cysteinyl-leukotriens (cys-LT), that could contribute to allergic/allergy-like side effects. Leukocytes from 39 patients receiving iopromide or iotrolan for routine CT-examination were analyzed for
Marc C Heinrich et al.
Radiology, 235(3), 843-849 (2005-04-23)
To compare the cytotoxic effects of dimeric and monomeric iodinated contrast media on renal tubular cells in vitro with regard to osmolality. LLC-PK1 cells were incubated with ioxithalamate, ioversol, iomeprol-300, iomeprol-150, iodixanol, iotrolan, and hyperosmolar mannitol solutions for 1-24 hours
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)