おすすめの製品
グレード
pharmaceutical primary standard
APIファミリー
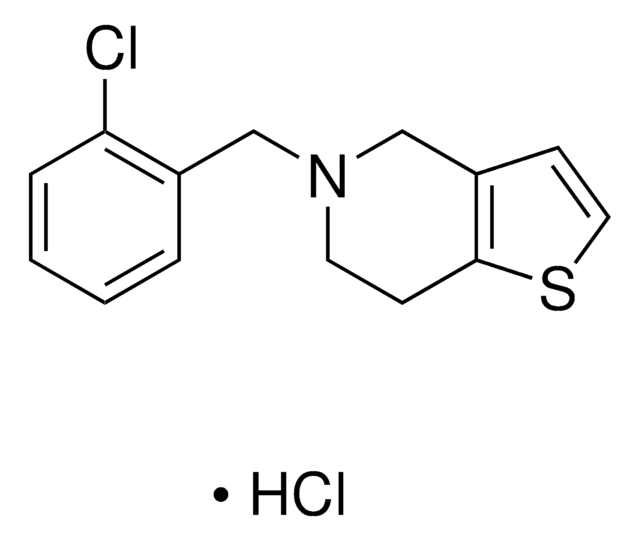
ticlopidine
メーカー/製品名
EDQM
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
SMILES記法
Cl.Clc1ccccc1CN2CCc3sccc3C2
InChI
1S/C14H14ClNS.ClH/c15-13-4-2-1-3-11(13)9-16-7-5-14-12(10-16)6-8-17-14;/h1-4,6,8H,5,7,9-10H2;1H
InChI Key
MTKNGOHFNXIVOS-UHFFFAOYSA-N
遺伝子情報
human ... P2RY12(64805)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
この製品は薬局方標準品です。発行元の薬局方により製造・供給されています。MSDSを含む製品情報などの詳しい情報は、発行元の薬局方のウェブサイトよりご確認ください。
アプリケーション
Ticlopidine hydrochloride EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包装
この製品は発行元の薬局方による供給としてお届けします。現在の単位量については、EDQM reference substance catalogueをご覧ください。
その他情報
Sales restrictions may apply.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
最新バージョンのいずれかを選択してください:
Morten Lamberts et al.
Circulation, 129(15), 1577-1585 (2014-01-29)
The optimal long-term antithrombotic treatment of patients with coexisting atrial fibrillation and stable coronary artery disease is unresolved, and commonly, a single antiplatelet agent is added to oral anticoagulation. We investigated the effectiveness and safety of adding antiplatelet therapy to
Stephen D Wiviott et al.
Lancet (London, England), 382(9892), 605-613 (2013-08-21)
Treatment with prasugrel and aspirin improves outcomes compared with clopidogrel and aspirin for patients with acute coronary syndrome who have had angiography and percutaneous coronary intervention; however, no clear benefit has been shown for patients managed first with drugs only.
Nohra Chalouhi et al.
Stroke, 45(1), 54-58 (2013-11-21)
Flow diverters are currently indicated for treatment of large and complex intracranial aneurysms. The purpose of this study was to determine whether the indications of flow diversion can be safely extended to unruptured, small, saccular aneurysms (<10 mm) of the
Julie A Johnson et al.
Pharmacological reviews, 65(3), 987-1009 (2013-05-21)
The past decade has seen tremendous advances in our understanding of the genetic factors influencing response to a variety of drugs, including those targeted at treatment of cardiovascular diseases. In the case of clopidogrel, warfarin, and statins, the literature has
Colin P Derdeyn et al.
Lancet (London, England), 383(9914), 333-341 (2013-10-31)
Early results of the Stenting and Aggressive Medical Management for Preventing Recurrent stroke in Intracranial Stenosis trial showed that, by 30 days, 33 (14·7%) of 224 patients in the stenting group and 13 (5·8%) of 227 patients in the medical
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)