おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to BP BP563
traceable to Ph. Eur. Y0001400
traceable to USP 1712001
CofA
current certificate can be downloaded
包装
pkg of 400 mg
アプリケーション
pharmaceutical
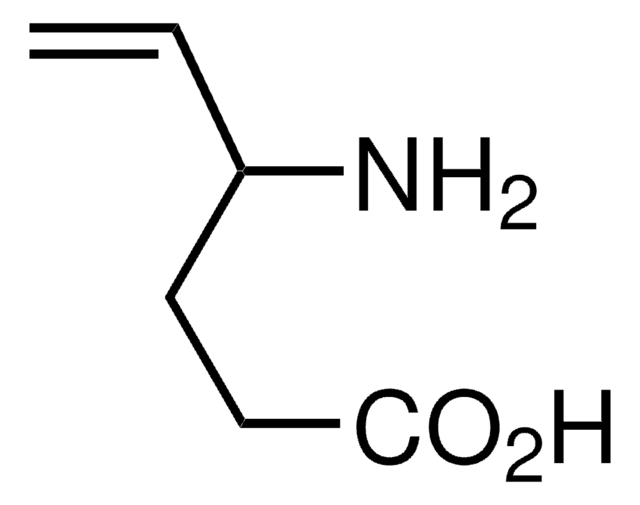
SMILES記法
NC(CCC(O)=O)C=C
InChI
1S/C6H11NO2/c1-2-5(7)3-4-6(8)9/h2,5H,1,3-4,7H2,(H,8,9)
InChI Key
PJDFLNIOAUIZSL-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Pharmaceutical secondary standards for application in quality control provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards
アプリケーション
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
生物化学的/生理学的作用
GABAトランスアミナ-ゼの不可逆性インヒビタ-です。神経終末のGABAの細胞内濃度を増加させます。抗てんかん作用を有しています。
アナリシスノート
These secondary standards offer multi-traceability to the USP, EP and BP primary standards, where they are available.
その他情報
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
脚注
To see an example of a Certificate of Analysis for this material enter LRAA6959(changes by product) in the Documents slot below. This is an example certificate only and may not be the lot that you receive.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
STOT RE 1
ターゲットの組織
Eyes,Central nervous system
保管分類コード
6.1D - Non-combustible acute toxic Cat.3 / toxic hazardous materials or hazardous materials causing chronic effects
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR3429-400MG:
最新バージョンのいずれかを選択してください:
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)