おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to Ph. Eur. Y0001281
traceable to USP 1287325
APIファミリー
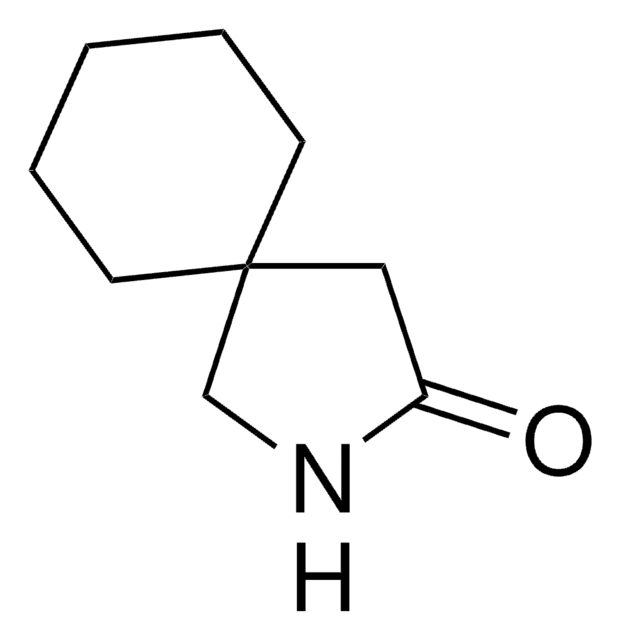
gabapentin
CofA
current certificate can be downloaded
テクニック
HPLC: suitable
gas chromatography (GC): suitable
mp
84-89 °C (lit.)
アプリケーション
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-8°C
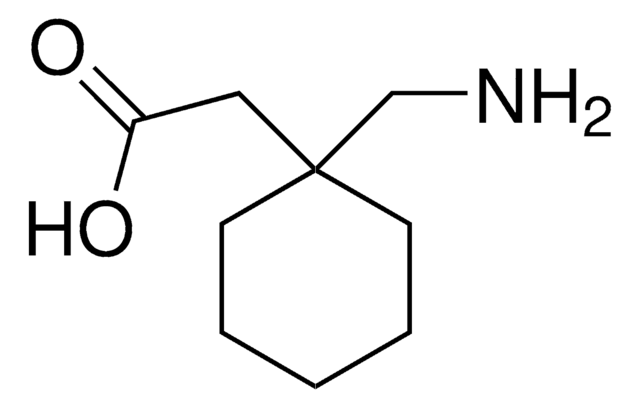
SMILES記法
O=C1CC2(CCCCC2)CN1
InChI
1S/C9H15NO/c11-8-6-9(7-10-8)4-2-1-3-5-9/h1-7H2,(H,10,11)
InChI Key
JAWPQJDOQPSNIQ-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
品質管理の認定を受けた製品番号医薬品の2次標準は、薬局方の1次標準に対する便利で対費用効果の高い代替品を医薬品研究室と製薬企業に提供します。
アプリケーション
Gabapentin Related Compound A may be used as a pharmaceutical reference standard for the determination of the analyte in pharmaceutical formulations by high performance liquid chromatography (HPLC).
These Secondary Standards are qualified as Certified Reference Materials. These are suitable for use in several analytical applications including but not limited to pharma release testing, pharma method development for qualitative and quantitative analyses, food and beverage quality control testing, and other calibration requirements.
アナリシスノート
このような2次標準は、USP、EP(PhEur)、BPの1次標準にマルチトレーサビリティを提供します。
その他情報
This Certified Reference Material (CRM) is produced and certified in accordance with ISO 17034 and ISO/IEC 17025. All information regarding the use of this CRM can be found on the certificate of analysis.
脚注
To see an example of a Certificate of Analysis for this material enter LRAC4059 in the slot below. This is an example certificate only and may not be the lot that you receive.
おすすめ製品
Find a digital Reference Material for this product available on our online platform ChemisTwin® for NMR. You can use this digital equivalent on ChemisTwin® for your sample identity confirmation and compound quantification (with digital external standard). An NMR spectrum of this substance can be viewed and an online comparison against your sample can be performed with a few mouseclicks. Learn more here and start your free trial.
関連製品
製品番号
詳細
価格
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1322-100MG-PW:
PHR1322-100MG:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
Development and validation of a stability-indicating RP-HPLC-CAD method for gabapentin and its related impurities in presence of degradation products
Ragham PK and Chandrasekhar KB
Journal of Pharmaceutical and Biomedical Analysis, 125, 122-129 (2016)
Sarah Al-Bachari et al.
The Cochrane database of systematic reviews, 7(7), CD001415-CD001415 (2013-07-28)
The majority of people with epilepsy have a good prognosis and their seizures are well controlled by a single antiepileptic drug, but up to 30% develop drug-resistant epilepsy, especially those with partial seizures. In this review we summarise the current
The intestinal absorption mechanism of gabapentin makes it appropriate for gastroretentive delivery.
Cuiping Chen et al.
Current clinical pharmacology, 8(1), 67-72 (2012-09-06)
Gabapentin is approved for the treatment of postherpetic neuralgia (PHN) and epilepsy. The pharmacokinetic (PK) properties of gabapentin, including absorption, distribution, metabolism, and excretion (ADME), were investigated during the development of Neurontin®, an immediate-release (IR) formulation of gabapentin that is
Lin Yu et al.
Spine, 38(22), 1947-1952 (2013-08-08)
Systematic review and meta-analysis. To review the literature systematically and make a comprehensive understanding of the efficacy of these 2 drugs in the management of postoperative pain after lumbar spinal surgery. Several trials that evaluated the efficacy of gabapentin and
Mattias Linde et al.
The Cochrane database of systematic reviews, 6(6), CD010609-CD010609 (2013-06-26)
Some antiepileptic drugs but not others are useful in clinical practice for the prophylaxis of migraine. This might be explained by the variety of actions of these drugs in the central nervous system. The present review is part of an
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)