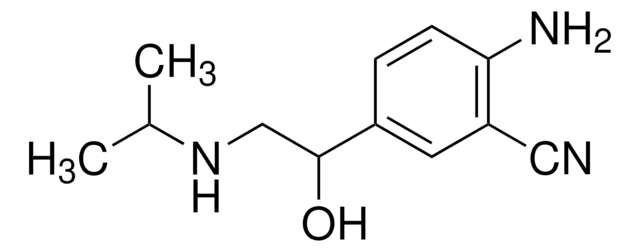
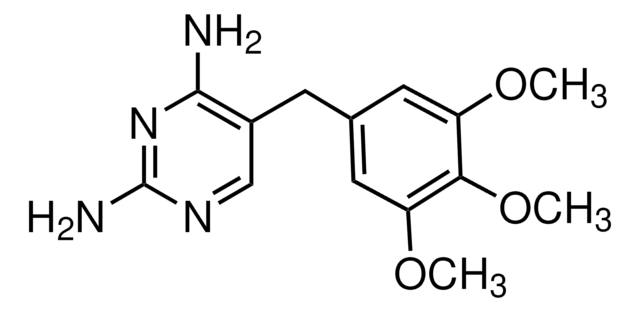
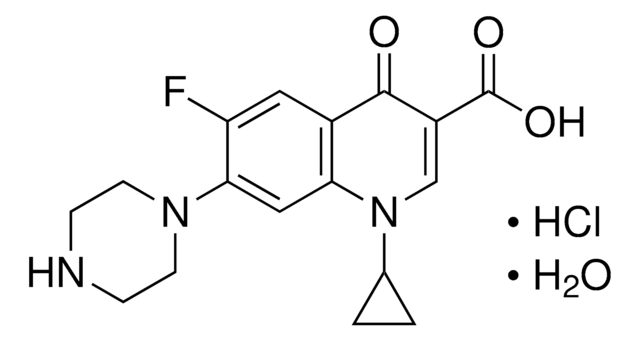
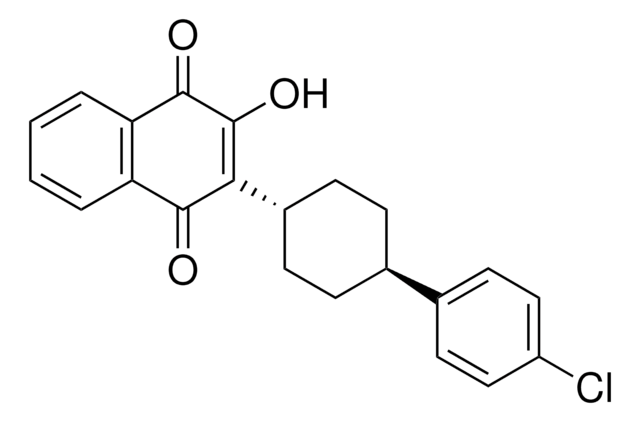
おすすめの製品
グレード
certified reference material
pharmaceutical secondary standard
品質水準
認証
traceable to USP 1464001
APIファミリー
nitrofurantoin
CofA
current certificate can be downloaded
テクニック
HPLC: suitable
gas chromatography (GC): suitable
アプリケーション
cleaning products
cosmetics
food and beverages
personal care
pharmaceutical (small molecule)
フォーマット
neat
保管温度
2-30°C
SMILES記法
[O-][N+](=O)c1ccc(\C=N\N2CC(=O)NC2=O)o1
InChI
1S/C8H6N4O5/c13-6-4-11(8(14)10-6)9-3-5-1-2-7(17-5)12(15)16/h1-3H,4H2,(H,10,13,14)/b9-3+
InChI Key
NXFQHRVNIOXGAQ-YCRREMRBSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
Nitrofurantoin, a synthetic, nitrofuran-derivative, is a widely used urinary antimicrobial compound that is effective against pulmonary fibrosis, neuropathy, hepatitis and hemolytic anemia.
アプリケーション
アナリシスノート
その他情報
脚注
関連製品
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Resp. Sens. 1 - Skin Sens. 1
保管分類コード
11 - Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
PHR1191-1G-PW:
PHR1191-1G:
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)