おすすめの製品
詳細
The analytes available for this multiplex kit are: Liver-type arginase 1 (ARG1), α-glutathianone S-transferase (GSTα), malate dehydrogenase 1 (MDH1), sorbitol dehydrogenase (SDH), and 5′-Nucleotidase (5′-NT/CD73).
品質水準
100
200
化学種の反応性
human
メーカー/製品名
Milliplex®
assay range
accuracy: 106-123%
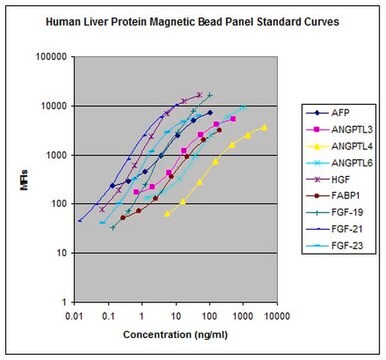
standard curve range: 14-10,000 pg/mL
(GSTα)
standard curve range: 41-30,000 pg/mL
(5′-NT/CD73)
standard curve range: 549-400,000 pg/mL
(SDH)
standard curve range: 69-50,000 pg/mL
(ARG1)
standard curve range: 69-50,000 pg/mL
(MDH1)
テクニック
multiplexing: suitable
検出方法
fluorometric (Luminex xMAP)
輸送温度
ambient
関連するカテゴリー
詳細
The MILLIPLEX® Human Liver Injury Panel contains all the components necessary to simultaneous quantify the following 5 analytes in serum and plasma samples:
• Liver-Type Arginase 1 (ARG1)*
• Malate dehydrogenase 1 (MDH1)*
• α-glutathione S-transferase (GSTα)*
• Sorbitol Dehydrogenase (SDH)*
• 5′-Nucleotidase/CD73 (5′-NT)
*ARG1, GSTα, and SDH are biomarkers listed in the Predictive Safety Testing Consortium (PSTC) project pipeline which have a strong translational role in drug safety testing.
The MILLIPLEX® portfolio offers the broadest selection of analytes across a wide range of disease states and species. Once the analytes of interest have been identified, you can rely on the quality that we build into each kit to produce results you can trust. In addition to the assay characteristics listed in the protocol, other performance criteria evaluated during the validation process include: cross-reactivity, dilution linearity, kit stability, and sample behavior (e.g. detectability and stability).
Panel Type: Toxicity
特異性
There was no or negligible cross-reactivity between the antibodies for an analyte and any of the other analytes within a panel.
アプリケーション
- Analytes: ARG1, GSTα, SDH 5′-NT/CD73, Malate dehydrogenase 1 (MDH1)
- Recommended Sample type: serum, plasma, and tissue culture supernatants
- Recommended Sample dilution: 1:5 serum or plasma samples. Tissue culture supernatants may require dilution as well.
- Assay Run Time: One day or Overnight
- Research Category: Toxicity
特徴および利点
包装
保管および安定性
その他情報
法的情報
免責事項
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 2 - Skin Sens. 1
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)