APT403
CaspaTag Caspase 3,7 In Situ Assay Kit, Fluorescein
The In Situ Caspase Detection Kit for Flow Cytometry use a novel approach to detect active caspases. The methodology is based on Fluorochrome Inhibitors of Caspases (FLICA).
ログイン組織・契約価格を表示する
すべての画像(1)
About This Item
UNSPSCコード:
12161503
eCl@ss:
32161000
NACRES:
NA.84
おすすめの製品
品質水準
化学種の反応性(ホモロジーによる予測)
mammals
メーカー/製品名
CaspaTag
Chemicon®
テクニック
activity assay: suitable
flow cytometry: suitable
NCBIアクセッション番号
検出方法
fluorometric
輸送温度
wet ice
遺伝子情報
human ... CASP3(836)
詳細
Apoptosis is an evolutionarily conserved form of cell suicide, which follows a specialized cellular process. The central component of this process is a cascade of proteolytic enzymes called caspases. These enzymes participate in a series of reactions that are triggered in response to pro-apoptotic signals and result in the cleavage of protein substrates, causing the disassembly of the cell (Slee et al. 1999).
Caspases have been identified in organisms ranging from C. elegans to humans. The mammalian caspases play distinct roles in apoptosis and inflammation. In apoptosis, caspases are responsible for proteolytic cleavages that lead to cell disassembly (effector caspases), and are involved in upstream regulatory events (initiator caspases). An active caspase consists of two large and two small subunits that form two heterodimers which associate in a tetramer (Walker et al. 1994; Wilson et al. 1994; Rotonda et al. 1996). In common with other proteases, caspases are synthesized as precursors that undergo proteolytic maturation, either autocatalytically or in a cascade by enzymes with similar specificity (Kumar 1999).
Caspase enzymes specifically recognize a 4 or 5 amino acid sequence on the target substrate which necessarily includes an aspartic acid residue. This residue is the target for cleavage, which occurs at the carbonyl end of the aspartic acid residue (Thornberry et al. 1997). Caspases can be detected via immunoprecipitation, immunoblotting techniques using caspase specific antibodies, or by employing fluorochrome substrates which become fluorescent upon cleavage by the caspase.
Test Principle:
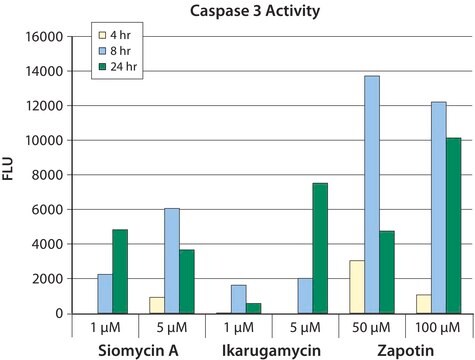
CHEMICON′s In Situ Caspase Detection Kits use a novel approach to detect active caspases. The methodology is based on Fluorochrome Inhibitors of Caspases (FLICA). The inhibitors are cell permeable and non-cytotoxic. Once inside the cell, the inhibitor binds covalently to the active caspase (Ekert et al. 1999). This kit uses a carboxyfluorescein-labeled fluoromethyl ketone peptide inhibitor of caspase-3 (FAM-DEVD-FMK), which produces a green fluorescence. When added to a population of cells, the FAM-DEVD-FMK probe enters each cell and covalently binds to a reactive cysteine residue that resides on the large subunit of the active caspase heterodimer, thereby inhibiting further enzymatic activity. The bound labeled reagent is retained within the cell, while any unbound reagent will diffuse out of the cell and is washed away. The green fluorescent signal is a direct measure of the amount of active caspase-3 or caspase-7 present in the cell at the time the reagent was added. Cells that contain the bound labeled reagent can be analyzed by 96-well plate-based fluorometry, fluorescence microscopy, or flow cytometry.
Application:
The CHEMICON In Situ FLICA Caspase-3/7 Detection Kit is a fluorescent-based assay for detection of active caspase-3 or caspase-7 in cells undergoing apoptosis. The kit is for research use only. Not for use in diagnostic or therapeutic procedures.
Caspases have been identified in organisms ranging from C. elegans to humans. The mammalian caspases play distinct roles in apoptosis and inflammation. In apoptosis, caspases are responsible for proteolytic cleavages that lead to cell disassembly (effector caspases), and are involved in upstream regulatory events (initiator caspases). An active caspase consists of two large and two small subunits that form two heterodimers which associate in a tetramer (Walker et al. 1994; Wilson et al. 1994; Rotonda et al. 1996). In common with other proteases, caspases are synthesized as precursors that undergo proteolytic maturation, either autocatalytically or in a cascade by enzymes with similar specificity (Kumar 1999).
Caspase enzymes specifically recognize a 4 or 5 amino acid sequence on the target substrate which necessarily includes an aspartic acid residue. This residue is the target for cleavage, which occurs at the carbonyl end of the aspartic acid residue (Thornberry et al. 1997). Caspases can be detected via immunoprecipitation, immunoblotting techniques using caspase specific antibodies, or by employing fluorochrome substrates which become fluorescent upon cleavage by the caspase.
Test Principle:
CHEMICON′s In Situ Caspase Detection Kits use a novel approach to detect active caspases. The methodology is based on Fluorochrome Inhibitors of Caspases (FLICA). The inhibitors are cell permeable and non-cytotoxic. Once inside the cell, the inhibitor binds covalently to the active caspase (Ekert et al. 1999). This kit uses a carboxyfluorescein-labeled fluoromethyl ketone peptide inhibitor of caspase-3 (FAM-DEVD-FMK), which produces a green fluorescence. When added to a population of cells, the FAM-DEVD-FMK probe enters each cell and covalently binds to a reactive cysteine residue that resides on the large subunit of the active caspase heterodimer, thereby inhibiting further enzymatic activity. The bound labeled reagent is retained within the cell, while any unbound reagent will diffuse out of the cell and is washed away. The green fluorescent signal is a direct measure of the amount of active caspase-3 or caspase-7 present in the cell at the time the reagent was added. Cells that contain the bound labeled reagent can be analyzed by 96-well plate-based fluorometry, fluorescence microscopy, or flow cytometry.
Application:
The CHEMICON In Situ FLICA Caspase-3/7 Detection Kit is a fluorescent-based assay for detection of active caspase-3 or caspase-7 in cells undergoing apoptosis. The kit is for research use only. Not for use in diagnostic or therapeutic procedures.
アプリケーション
Research Category
アポトーシス及び癌
アポトーシス及び癌
The In Situ Caspase Detection Kit for Flow Cytometry use a novel approach to detect active caspases. The methodology is based on Fluorochrome Inhibitors of Caspases (FLICA).
構成
FLICA Reagent (FAM-DEVD-FMK): Four lyophilized vials
10X Wash Buffer: 60 mL
Fixative: 6 mL
Propidium Iodide: 1 mL at 250 μg/mL, ready-to-use
Hoechst Stain: 1 mL at 200 μg/mL, ready-to-use
10X Wash Buffer: 60 mL
Fixative: 6 mL
Propidium Iodide: 1 mL at 250 μg/mL, ready-to-use
Hoechst Stain: 1 mL at 200 μg/mL, ready-to-use
保管および安定性
· Store unopened kit materials at 2-8°C up to their expiration date.
· Reconstituted FLICA Reagent (150X) should be frozen at -20ºC for up to 6 months, and may be thawed twice during this time. Aliquot into separate amber tubes if desired. Protect from light at all times.
· Store diluted (1X) wash buffer up to -20ºC for 2 weeks.
· Reconstituted FLICA Reagent (150X) should be frozen at -20ºC for up to 6 months, and may be thawed twice during this time. Aliquot into separate amber tubes if desired. Protect from light at all times.
· Store diluted (1X) wash buffer up to -20ºC for 2 weeks.
法的情報
CHEMICON is a registered trademark of Merck KGaA, Darmstadt, Germany
免責事項
Unless otherwise stated in our catalog or other company documentation accompanying the product(s), our products are intended for research use only and are not to be used for any other purpose, which includes but is not limited to, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses or any type of consumption or application to humans or animals.
シグナルワード
Danger
危険有害性の分類
Acute Tox. 3 Inhalation - Acute Tox. 4 Dermal - Acute Tox. 4 Oral - Carc. 1B - Eye Irrit. 2 - Muta. 2 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 2 - STOT SE 3
ターゲットの組織
Eyes,Central nervous system, Respiratory system
保管分類コード
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
APT403:
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
Qunfeng Cai et al.
Neurobiology of disease, 45(2), 723-732 (2011-11-03)
Cell-cell junctions and junctions between cells and extracellular matrix are essential for maintenance of the structural and functional integrity of the cochlea, and are also a major target of acoustic trauma. While morphological assessments have revealed adhesion dysfunction in noise-traumatized
Bo Hua Hu et al.
Journal of neuroscience research, 88(8), 1812-1821 (2010-01-22)
Acoustic overstimulation causes apoptotic cell death in the cochlea. This death process is mediated, in part, by the mitochondrial signaling pathway involving Bcl-2 family proteins. Myeloid cell leukemia sequence 1 (Mcl-l) is an antiapoptotic member of the Bcl-2 family. Its
Lisa L Cunningham et al.
The Journal of neuroscience : the official journal of the Society for Neuroscience, 22(19), 8532-8540 (2002-09-28)
Aminoglycoside exposure results in the apoptotic destruction of auditory and vestibular hair cells. This ototoxic hair cell death is prevented by broad-spectrum caspase inhibition. We have used in situ substrate detection, immunohistochemistry, and specific caspase inhibitors to determine which caspases
Mohammad Husein Abnosi et al.
Iranian journal of basic medical sciences, 15(4), 900-906 (2013-03-16)
Arsenic compounds are potent human carcinogen and produce a variety of stress responses in mammalian cells. Recently sodium arsenite has been recommended to be used as anti malignancy drug by American food and drug administration (FDA). In this study, we
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)