おすすめの製品
品質水準
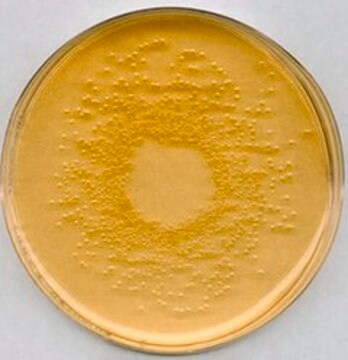
無菌性
sterile
形状
plate
特徴
ready-to-use
ICR Settle plate
包装
box of 120 plates
テクニック
microbiological culture: suitable
plate diam. × filling volume
90 mm × 20 mL
pH
7 ( in H2O)
アプリケーション
bioburden testing
industrial qc
life science and biopharma
microbiology
pharmaceutical
保管温度
15-25°C
詳細
Minimal Glucose Agar is used for the microbial mutagenicity Ames test. It is a bacterial bioassay performed in vitro to evaluate the mutagenicity of various environmental carcinogens and toxins. The principle is to detect point mutations using amino acid requiring strains. The Minimal Glucose Agar plate is taken as a bottom agar for the mutagenicity assay and includes the minimum ingredients for bacterial growth. Phosphates act as the buffering system. Glucose and Citrate are the carbon sources to produce energy. Magnesium is a cofactor for many metabolic reactions
アプリケーション
Minimal Glucose Agar for Ames test is used to identify the revert mutations which are present in strains. It can also be used to detect the mutagenicity of environmental samples such as drugs, dyes, reagents, cosmetics, wastewater, pesticides, and other substances which are easily solubilized in a liquid suspension.
保管分類コード
13 - Non Combustible Solids
WGK
WGK 1
引火点(°F)
Not applicable
引火点(℃)
Not applicable
試験成績書(COA)
製品のロット番号・バッチ番号を入力して、試験成績書(COA) を検索できます。ロット番号・バッチ番号は、製品ラベルに「Lot」または「Batch」に続いて記載されています。
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)