おすすめの製品
グレード
certified reference material
フォーム
liquid
特徴
Snap-N-Spike®/Snap-N-Shoot®
包装
ampule of 1 mL
メーカー/製品名
Cerilliant®
濃度
100 μg/mL in methanol
テクニック
gas chromatography (GC): suitable
liquid chromatography (LC): suitable
アプリケーション
clinical testing
フォーマット
single component solution
保管温度
−20°C
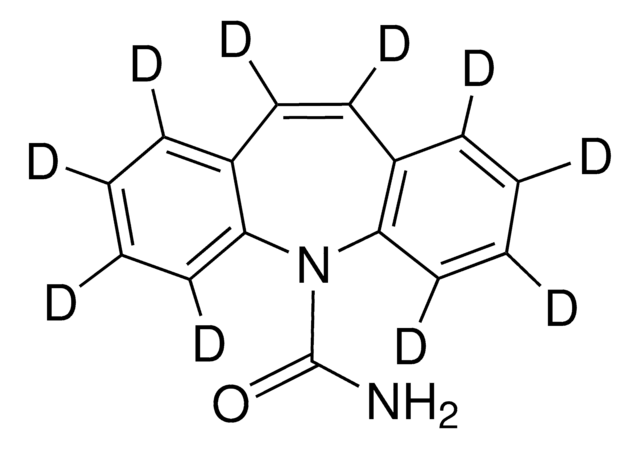
SMILES記法
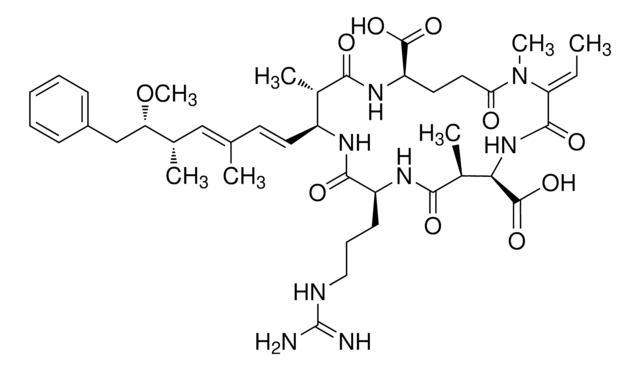
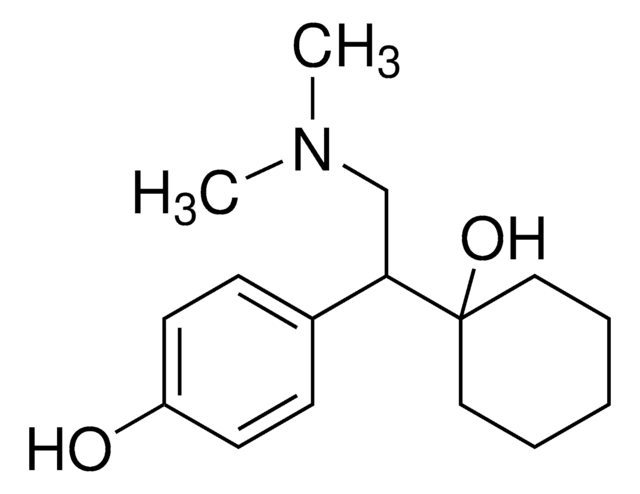
OC1=CC=C(C(CN(C)C)C2(O)CCCCC2)C=C1
InChI
1S/C16H25NO2/c1-17(2)12-15(13-6-8-14(18)9-7-13)16(19)10-4-3-5-11-16/h6-9,15,18-19H,3-5,10-12H2,1-2H3
InChI Key
KYYIDSXMWOZKMP-UHFFFAOYSA-N
遺伝子情報
human ... SLC6A2(6530) , SLC6A4(6532)
類似した製品をお探しですか? 訪問 製品比較ガイド
詳細
法的情報
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Flam. Liq. 2 - STOT SE 1
ターゲットの組織
Eyes
保管分類コード
3 - Flammable liquids
WGK
WGK 1
引火点(°F)
49.5 °F - closed cup
引火点(℃)
9.7 °C - closed cup
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
アルコール類
危険等級II
Jan Code
V-007-CC:
V-007-1ML:
最新バージョンのいずれかを選択してください:
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)