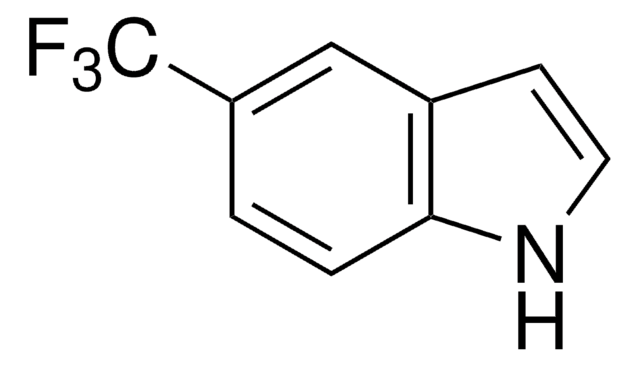
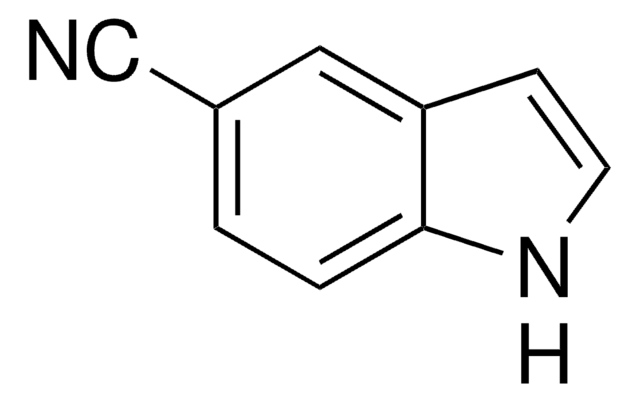
おすすめの製品
品質水準
アッセイ
99%
bp
176-178 °C/17 mmHg (lit.)
mp
52-55 °C (lit.)
SMILES記法
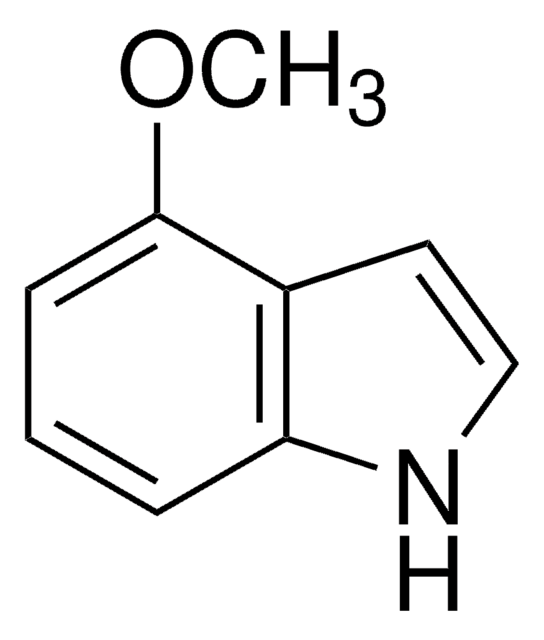
COc1ccc2[nH]ccc2c1
InChI
1S/C9H9NO/c1-11-8-2-3-9-7(6-8)4-5-10-9/h2-6,10H,1H3
InChI Key
DWAQDRSOVMLGRQ-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
M14900-5G:
M14900-VAR:
M14900-BULK:
M14900-1G:
M14900-5KG:
この製品を見ている人はこちらもチェック
Christian Brand et al.
The Journal of chemical physics, 133(2), 024303-024303 (2010-07-17)
Rotationally resolved electronic spectra of the vibrationless origin and of eight vibronic bands of 5-methoxyindole (5MOI) have been measured and analyzed using an evolutionary strategy approach. The experimental results are compared to the results of ab initio calculations. All vibronic
Kenichiro Todoroki et al.
Analytical sciences : the international journal of the Japan Society for Analytical Chemistry, 22(2), 281-286 (2006-03-04)
In this paper we describe a highly sensitive and selective liquid chromatographic method for the determination of 5-methoxyindoles (5-methoxyindole-3-acetic acid, 5-methoxytryptamine, 5-methoxytryptophol, and melatonin) using a post-column technique involving electrolytic demethylation followed by fluorescence derivatization with benzylamine. We separated these
Pawel Sledz et al.
Journal of the American Chemical Society, 132(13), 4544-4545 (2010-03-18)
Fragment-based methods are a new and emerging approach for the discovery of protein binders that are potential new therapeutic agents. Several ways of utilizing structural information to guide the inhibitor assembly have been explored to date. One of the approaches
F Peter Guengerich et al.
Journal of medicinal chemistry, 47(12), 3236-3241 (2004-05-28)
Indigoids, a class of bis-indoles, represent a promising protein kinase inhibitor scaffold. Oxidation of indole by cytochrome P450 (P450) has been shown to generate species (indoxyl, isatin) that couple to yield indigo and indirubin. Escherichia coli-expressed human P450 2A6 mutants
Xiang-Qun Hu et al.
The Journal of biological chemistry, 283(11), 6826-6831 (2008-01-12)
Current receptor theory suggests that there is an equilibrium between the inactive (R) and active (R*) conformations of ligand-gated ion channels and G protein-coupled receptors. The actions of ligands in both receptor types could be appropriately explained by this two-state
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)