おすすめの製品
品質水準
アッセイ
98%
フォーム
powder
mp
124-128 °C (lit.)
SMILES記法
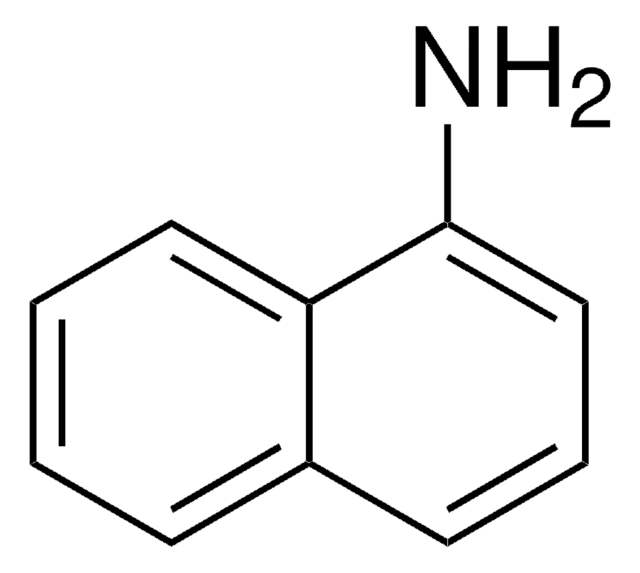
Nc1ccc-2c(Cc3ccccc-23)c1
InChI
1S/C13H11N/c14-11-5-6-13-10(8-11)7-9-3-1-2-4-12(9)13/h1-6,8H,7,14H2
InChI Key
CFRFHWQYWJMEJN-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Aquatic Chronic 2 - Carc. 2
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Eyeshields, Gloves, type N95 (US)
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
A55500-1KG:
A55500-5G:
A55500-25G:
A55500-BULK:
A55500-VAR:
この製品を見ている人はこちらもチェック
R H Heflich et al.
Mutation research, 318(2), 73-114 (1994-10-01)
2-Acetylaminofluorene and 2-aminofluorene are among the most intensively studied of all chemical mutagens and carcinogens. Fundamental research findings concerning the metabolism of 2-acetylaminofluorene to electrophilic derivatives, the interaction of these derivatives with DNA, and the carcinogenic and mutagenic responses that
Stephen W Holman et al.
Rapid communications in mass spectrometry : RCM, 22(15), 2355-2365 (2008-07-10)
A 50 m/z unit loss from protonated 4-benzenesulfinyl-3-methylphenylamine has been observed and investigated using electrospray ionisation quadrupole ion trap mass spectrometry (ESI-QIT-MS). It was hypothesised that the specific fragmentation was affected by the presence of an ortho methyl group in
Dominique Y Burnouf et al.
Journal of molecular biology, 386(4), 951-961 (2009-01-20)
The model carcinogen N-2-acetylaminofluorene covalently binds to the C8 position of guanine to form two adducts, the N-(2'-deoxyguanosine-8-yl)-aminofluorene (G-AF) and the N-2-(2'-deoxyguanosine-8-yl)-acetylaminofluorene (G-AAF). Although they are chemically closely related, their biological effects are strongly different and they are processed by
Jun Zhao et al.
Stem cell research & therapy, 10(1), 165-165 (2019-06-15)
Mounting evidence has shown that a novel subset of mesenchymal stem cells (MSCs) derived from human gingiva referred to as gingival mesenchymal stem cells (GMSCs) displays a greater immunotherapeutic potential and regenerative repair expression than MSCs obtained from other tissues.
Olga Rechkoblit et al.
Nature structural & molecular biology, 17(3), 379-388 (2010-02-16)
The aromatic amine carcinogen 2-aminofluorene (AF) forms covalent adducts with DNA, predominantly with guanine at the C8 position. Such lesions are bypassed by Y-family polymerases such as Dpo4 via error-free and error-prone mechanisms. We show that Dpo4 catalyzes elongation from
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)