おすすめの製品
詳細
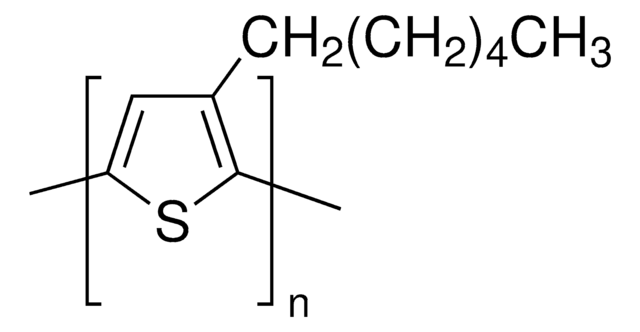
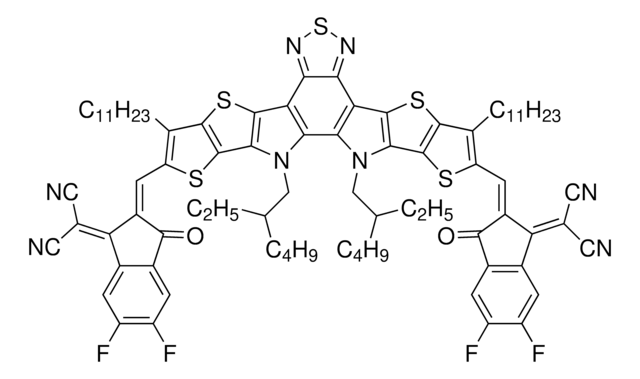
Band gap: 1.48 eV
アッセイ
98%
形状
powder
軌道エネルギー
HOMO -5.68 eV
LUMO -4.20 eV
詳細
アプリケーション
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
毒物及び劇物取締法
劇物
Jan Code
906387-BULK:
906387-VAR:
906387-100MG:4548173358314
最新バージョンのいずれかを選択してください:
この製品を見ている人はこちらもチェック
資料
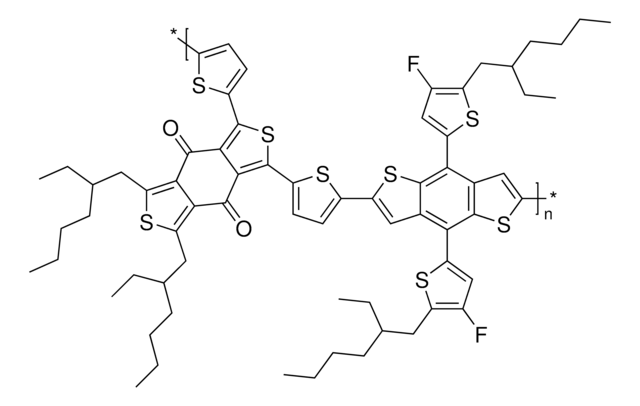
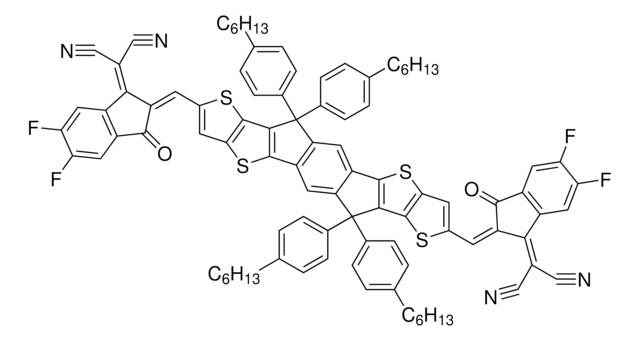
The emerging organic photovoltaic (OPV) technology is very promising for low-cost solar energy production. OPV devices can be produced using high-throughput, large-volume printing methods on lightweight and flexible plastic substrates, making them easy to deploy and use in innovative ways.
The emerging organic photovoltaic (OPV) technology is very promising for low-cost solar energy production.
Professor Chen (Nankai University, China) and his team explain the strategies behind their recent record-breaking organic solar cells, reaching a power conversion efficiency of 17.3%.
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)


![[6,6]-フェニル C71 酪酸メチルエステル、異性体混合物 99%](/deepweb/assets/sigmaaldrich/product/structures/716/624/9fb9f2f0-ae99-429f-8d3a-b12267976a4d/640/9fb9f2f0-ae99-429f-8d3a-b12267976a4d.png)