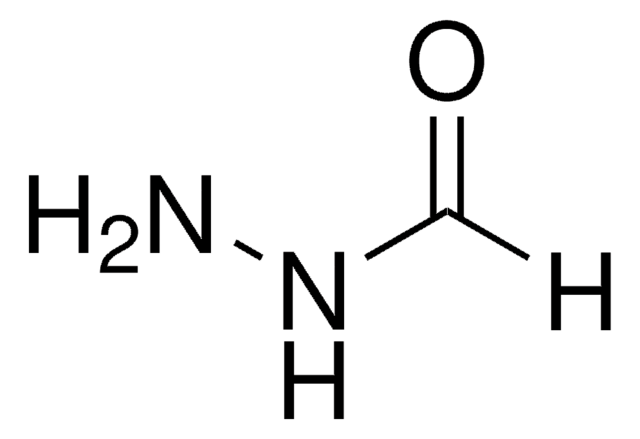
おすすめの製品
アッセイ
≥95%
品質水準
フォーム
powder
分子量
229.32
反応適合性
reagent type: cross-linking reagent
保管条件
desiccated
溶解性
DMSO or DMF: soluble
官能基
hydrazide
輸送温度
ambient
保管温度
2-8°C
SMILES記法
NNC(CCSSC1=NC=CC=C1)=O
InChI
1S/C8H11N3OS2/c9-11-7(12)4-6-13-14-8-3-1-2-5-10-8/h1-3,5H,4,6,9H2,(H,11,12)
InChI Key
NITXODYAMWZEJY-UHFFFAOYSA-N
関連するカテゴリー
詳細
The PDPH is a heterobifunctional crosslinker containing sulfhydryl-reactive pyridyldithiol and carbonyl-reactive hydrazide moieties. Pyridyldithiols react with free sulfhydryls (-SH) to form disulfide bonds. Hydrazide groups react with carbonyls (aldehydes and ketones) to form stable hydrazone bonds. Aldehyde groups can be created by periodate-oxidation of sialic acid and other sugar components of glycoprotein polysaccharides. Thus, PDPH is useful for conjugating glycoproteins and sulfhydryl-containing peptides or proteins. Likewise, PDPH is useful as a sulfhydryl-addition reagent for glycoproteins and other carbohydrates; after reaction of the hydrazide to an oxidized carbohydrate, the pyridyldithiol group can be cleaved by a reducing agent to expose a sulfhydryl group. Yet another application for PDPH is to react the primary amine of the hydrazide moiety to a carboxyl group using the crosslinker EDC.
特徴および利点
- Reactive groups: pyridyldisulfide and hydrazide
- Reactive toward: sulfhydryl groups and carbonyl (aldehyde) groups
- Short (9.2A), sulfhydryl-to-aldehyde crosslinker with disulfide bond spacer arm (cleavable)
- Pyridyldithiol group results in attachment to sulfhydryls via disulfide bond, which can be cleaved with DTT, TCEP or other reducing agents
- Hydrazide group conjugates to oxidized sugars of glycoproteins and carbohydrates
- Use sodium meta-periodate to oxidize glycosylation (e.g., sialic acid) to reactive aldehyde groups
- Use with EDC to conjugate primary amine of hydrazide group to carboxyl groups
注意
This product is sensitive to moisture. The vial is packaged in a resealable bag with a desiccant to reduce exposure to moisture. After cold storage, equilibrate the vial to room temperature before opening to reduce condensation inside the vial. Make fresh solutions. Storage of stock solutions is not recommended. After use, return the vial to the resealable bag. Close the bag and store the product at the recommended temperature.
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
803480-50MG:
最新バージョンのいずれかを選択してください:
D Pain et al.
Journal of immunological methods, 40(2), 219-230 (1981-01-01)
Protein A-peroxidase monoconjugate was prepared in solution using a heterobifunctional reagent, N-succinimidyl-3-(2-pyridyldithio)-propionate. The yield of the monoconjugate was much higher than that obtained with current methods. An immunoassay method was developed in which protein A-peroxidase monoconjugate served as a universal
G N Ranadive et al.
Nuclear medicine and biology, 20(6), 719-726 (1993-08-01)
We have developed a very efficient labeling technique for monoclonal antibodies with technetium-99m. Oxidation of sugar residues on the IgG class of antibodies leads to the generation of aldehyde groups which are further reacted with two newly developed hydrazide compounds.
D J O'Shannessy et al.
Immunology letters, 8(5), 273-277 (1984-01-01)
A novel method is described for the biotinylation of immunoglobulins. The procedure relies on the generation of reactive aldehydes on the carbohydrate moieties of the immunoglobulin by oxidation with sodium periodate and subsequent reaction with biotin hydrazide. The method is
Immobilization of glycoconjugates by their oligosaccharides: use of hydrazido-derivatized matrices.
D J O'Shannessy et al.
Analytical biochemistry, 191(1), 1-8 (1990-11-15)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)
![LC-SPDP (succinimidyl 6-[3(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/300/586/d95fd80c-e201-4b0b-8aee-31e109c2ff41/640/d95fd80c-e201-4b0b-8aee-31e109c2ff41.png)

![Sulfo-LC-SPDP (sulfosuccinimidyl 6-[3′-(2-pyridyldithio)propionamido]hexanoate)](/deepweb/assets/sigmaaldrich/product/structures/266/633/e2a263be-4bd3-4fcf-89c4-75b5e2bd829c/640/e2a263be-4bd3-4fcf-89c4-75b5e2bd829c.png)