すべての画像(1)
About This Item
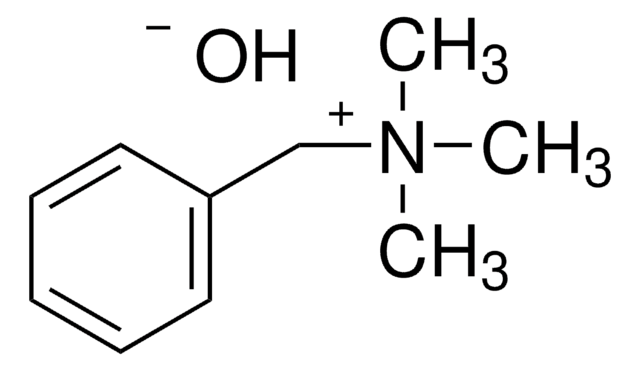
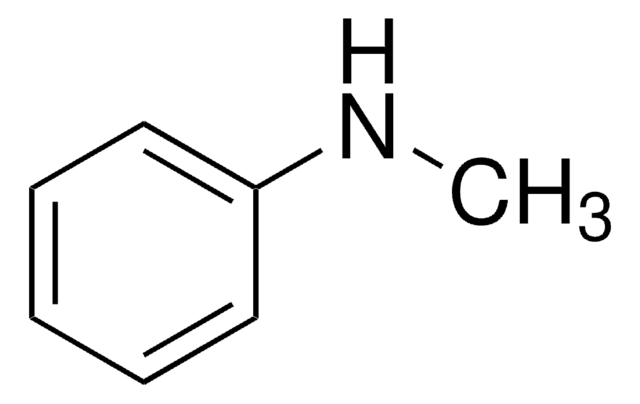
化学式:
(CH3)3N(OH)C6H5
CAS番号:
分子量:
153.22
Beilstein:
3917033
MDL番号:
UNSPSCコード:
12352005
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
フォーム
liquid
濃度
~25% in H2O (1.68 M)
屈折率
n20/D 1.395
官能基
amine
SMILES記法
[OH-].C[N+](C)(C)c1ccccc1
InChI
1S/C9H14N.H2O/c1-10(2,3)9-7-5-4-6-8-9;/h4-8H,1-3H3;1H2/q+1;/p-1
InChI Key
HADKRTWCOYPCPH-UHFFFAOYSA-M
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
Trimethylphenylammonium hydroxide solution (TMPAH) is a quaternary ammonium compound that is commonly used as a strong base in organic synthesis. It is also used as a deprotecting agent for the removal of t-butoxycarbonyl (Boc) groups from amino acids or peptides and benzyl protecting groups from alcohols or amines.
アプリケーション
Trimethylphenylammonium hydroxide solution can be used to initiate the polymerization of the monomer 1,8-dihydroxymethyl-1,3,5,7-octatetrayne (DHOT) for the preparation of polymer nanospheres. It is also used as a base in the synthesis of alkyl 3-nitroacrylates via aldol reaction with nitroacetaldehyde or nitroacetone.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Eye Dam. 1 - Skin Corr. 1B
保管分類コード
8A - Combustible corrosive hazardous materials
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
79267-VAR:
79267-100ML:
79267-500ML:
79267-BULK:
この製品を見ている人はこちらもチェック
Fazel Abdolahpur Monikh et al.
Nature communications, 12(1), 899-899 (2021-02-11)
Analytical limitations considerably hinder our understanding of the impacts of the physicochemical properties of nanomaterials (NMs) on their biological fate in organisms. Here, using a fit-for-purpose analytical workflow, including dosing and emerging analytical techniques, NMs present in organisms are characterized
R W Gullick et al.
Environmental science & technology, 35(7), 1523-1530 (2001-05-12)
A natural shale and four synthetic organoclays were compared as potential sorbent additives to containment barriers at hazardous waste sites. Trimethylphenyl ammonium bentonite (TMPA-bent) was shown in batch experiments to have the greatest sorption capacities for 1,2,4-trichlorobenzene, trichloroethylene, and methyl
Jeffrey T Auletta et al.
Chemico-biological interactions, 187(1-3), 135-141 (2010-05-25)
Acetylcholinesterase (AChE) contains a narrow and deep active site gorge with two sites of ligand binding, an acylation site (or A-site) at the base of the gorge and a peripheral site (or P-site) near the gorge entrance. The P-site contributes
Anthony A Mikulec et al.
Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 30(2), 131-138 (2009-01-31)
Drugs applied to the middle ear enter perilymph through the bony otic capsule. Drugs applied intratympanically in humans are thought to enter the cochlea primarily through the round window membrane (RWM). Local drug treatments of the ear are commonly evaluated
Alec N Salt et al.
Journal of the Association for Research in Otolaryngology : JARO, 13(6), 771-783 (2012-09-13)
Perilymph pharmacokinetics was investigated by a novel approach, in which solutions containing drug or marker were injected from a pipette sealed into the perilymphatic space of the lateral semi-circular canal (LSCC). The cochlear aqueduct provides the outlet for fluid flow
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)