おすすめの製品
品質水準
アッセイ
97%
フォーム
solid
mp
139-141 °C (lit.)
官能基
hydroxyl
SMILES記法
CC(C)(C)c1cc(CO)cc(c1O)C(C)(C)C
InChI
1S/C15H24O2/c1-14(2,3)11-7-10(9-16)8-12(13(11)17)15(4,5)6/h7-8,16-17H,9H2,1-6H3
InChI Key
HNURKXXMYARGAY-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
関連するカテゴリー
詳細
3,5-di-tert-butyl-4-hydroxybenzyl alcohol also known as 4-Hydroxymethyl-2,6-di-tert-butylphenol that is commonly used as an antioxidant in the preparation of PMA-type bifunctional polymers.
アプリケーション
3,5-Di-tert-butyl-4-hydroxybenzyl alcohol can be used as a reactant to synthesize:
- 2,6-di-tert-butyl-4-(dodecylselanylmethyl)phenol and bis(3,5-di-tert-butyl-4-hydroxybenzyl) selenide by reacting with dodecaneselenolate and sodium selenide.
- Monomeric antioxidant by reacting with imidazole and N-[4-(chlorocarbonyl)phenyl]maleimide.
- Sulfur-containing butylated hydroxytoluene derivatives by reacting with aryl/alky dithiols.
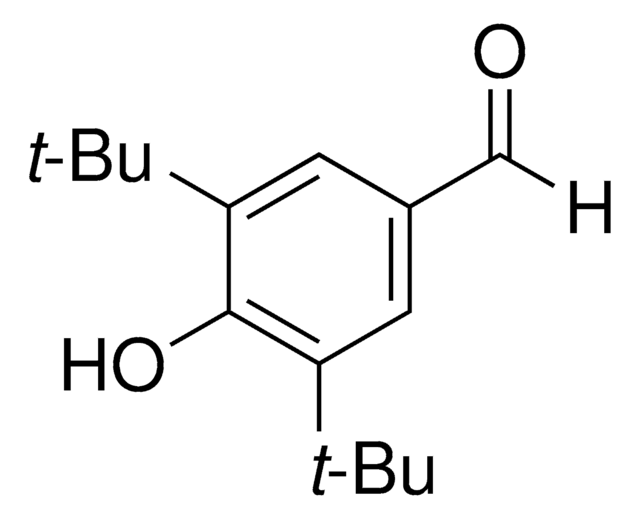
- 3,5-Di-tert-butyl-4-hydroxybenzaldehyde by oxidation reaction using stabilized IBX.
危険有害性情報
注意書き
危険有害性の分類
Aquatic Chronic 3
保管分類コード
11 - Combustible Solids
WGK
WGK 3
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
446424-BULK:
446424-VAR:
446424-25G:
446424-5G:
この製品を見ている人はこちらもチェック
O L Brekke et al.
Cytokine, 4(4), 269-280 (1992-07-01)
The effect of commonly used food antioxidants on recombinant tumor necrosis factor alpha (rTNF-alpha)-induced cytotoxicity, growth enhancement and adhesion has been evaluated. Butylated hydroxyanisole (BHA) and 4-hydroxymethyl-2,6-di-t-butylphenol (HBP) were the only two of nine antioxidants that completely inhibited rTNF-alpha-induced cytotoxicity
L R Barclay et al.
Biochimica et biophysica acta, 1328(1), 1-12 (1997-08-14)
Phenolic antioxidants of the hydroxychroman class, alpha-tocopherol (alpha-TOC) and 2,2,5,6,7-pentamethyl-6-hydroxychroman (PMHC), and the hindered phenols 2,3-dihydro-5-hydroxy-2,2,4-trimethylnaphtho[1,2-b]furan (NFUR), 2,6-di-tert-butyl-4-methoxyphenol (DBHA), and 2,6-di-tert-butyl-4-methyl phenol (BHT), were delivered into oxidizable (ACCEPTOR) liposomes of dilinoleoylphosphatidylcholine (DLPC) or 1-palmitoyl-2-linoleoyl-phosphatidylcholine (PLPC) from saturated DONOR liposomes of
REACTION OF SEVEN-AND EIGHT-MEMBERED CYCLIC PHOSPHOROCHLORIDITES WITH 3, 5-DI-tert-BUTYL-4-HYDROXYBENZYL ALCOHOL: FACILE P [sbnd] C BOND FORMATION.
Odorisio PA, et al.
Phosph. Sulfur Relat. Elem., 20(3), 273-277 (1984)
The antioxidant activity of 3, 5-di-tert-butyl-4-hydroxybenzyl derivatives.
Kim DH and Kummerow FA.
Journal of the American Chemical Society, 39(3), 150-155 (1962)
Synthesis of new polymeric antioxidants.
Oh DR, et al.
Bull. Korean Chem. Soc., 22(6), 629-632 (2001)
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)