すべての画像(1)
About This Item
実験式(ヒル表記法):
C8H10
CAS番号:
分子量:
106.17
Beilstein:
1616308
MDL番号:
UNSPSCコード:
12352100
PubChem Substance ID:
NACRES:
NA.22
おすすめの製品
詳細
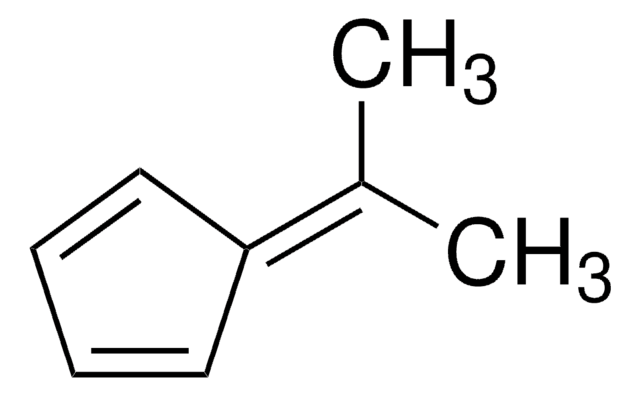
6,6-Dimethylfulvene [5-(1-methylethylidene)-1,3-cyclopentadiene] is a nonaromatic carbocyclic analog of isopropylbenzene. 6,6-Dimethylfulvene reacts with 2,2-bis(trifluoromethyl)-1,1-dicyanoethylene (BTF; 1) to afford the expected Diels-Alder cycloadduct, 7-(1-methylethylidene)-3,3-bis(trifluoromethyl)bicyclo[2.2.1]hept-5-ene-2,2-dicarbonitrile. Metal-free hydrogenation of 6,6-dimethylfulvene via frustrated Lewis pair (FLP) mediated triple domino reaction has been reported.The biotransformation of 6,6-dimethylfulvene by Pseudomonas putida RE213 has been studied.Cycloaddition of 6,6-dimethylfulvene with benzynes has been reported.
6,6-Dimethylfulvene undergoes nucleophilic reaction with lithium dichloromethide in the presence of THF at -75°C, selectively at its exocyclic double bond.
アプリケーション
6,6-Dimethylfulvene may be employed in the following studies:
- One-pot synthesis of ansa-metallocenes.
- Synthesis of endo and exo-adducts with maleic anhydride.
- Synthesis of fulvenols or the corresponding trimethylsilyl ethers.
シグナルワード
Danger
危険有害性情報
危険有害性の分類
Asp. Tox. 1 - Flam. Liq. 3
保管分類コード
3 - Flammable liquids
WGK
WGK 3
引火点(°F)
109.4 °F - closed cup
引火点(℃)
43 °C - closed cup
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第二石油類
危険等級III
非水溶性液体
Jan Code
40288-VAR:
40288-5ML:
40288-BULK:
この製品を見ている人はこちらもチェック
One-Step Synthesis of Organolanthanide (II) Complexes from the Metal.
Recknagel A and Edelmann FT.
Angewandte Chemie (International Edition in English), 30(6), 693-694 (1991)
Functionalization of 6, 6-Dimethylfulvene: A Fulvene Analogue of the Aldol Condensation.
Nystrom JE, et al.
Tetrahedron Letters, 29(39), 4997-5000 (1988)
Michael H Howard et al.
The Journal of organic chemistry, 68(1), 120-129 (2003-01-08)
Reaction of 2,2-bis(trifluoromethyl)-1,1-dicyanoethylene (BTF; 1) with 6,6-dimethylfulvene (2) affords the expected Diels-Alder cycloadduct, 7-(1-methylethylidene)-3,3-bis(trifluoromethyl)bicyclo[2.2.1]hept-5-ene-2,2-dicarbonitrile (3), in good yield. The cycloadduct 3 is unstable and exists in equilibrium with the starting materials in less polar solvents. In more polar environment, the
Sergej Tamke et al.
Organic & biomolecular chemistry, 12(45), 9139-9144 (2014-10-09)
The frustrated Lewis pair (FLP) mediated hydrosilylation of pentafulvenes is described yielding allyl silanes with high regioselectivity in excellent yields. While phenyl substituted allyl silanes undergo B(C6F5)3-mediated rearrangement to vinyl silanes, dimethyl derivatives experience FLP-catalyzed hydrogenation followed by an unprecedented
R W Eaton et al.
Applied and environmental microbiology, 62(3), 756-760 (1996-03-01)
The biotransformation of 6,6-dimethylfulvene [5-(1-methylethylidene)-1,3-cyclopentadiene], a nonaromatic C(inf5) carbocyclic analog of isopropylbenzene, was examined by using Pseudomonas putida RE213, a Tn5-generated dihydrodiol-accumulating mutant of the isopropylbenzene-degrading strain P. putida RE204. 6,6-Dimethylfulvene was converted to a single chiral product identified as
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)