おすすめの製品
アッセイ
97%
mp
254-255 °C (lit.)
官能基
carboxylic acid
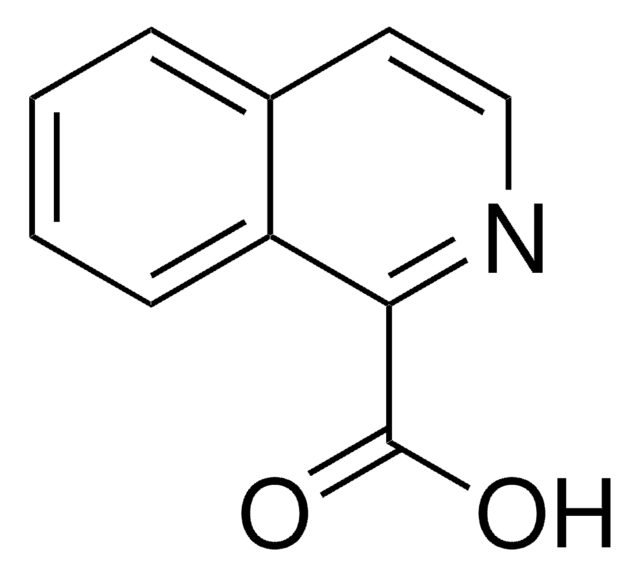
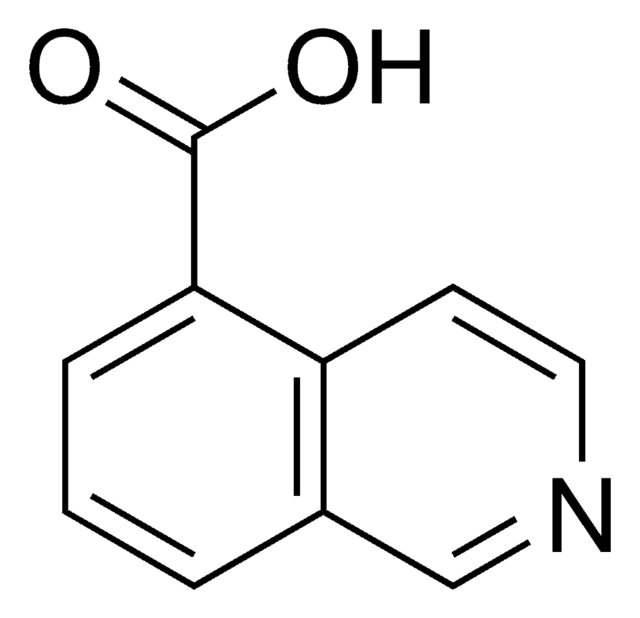
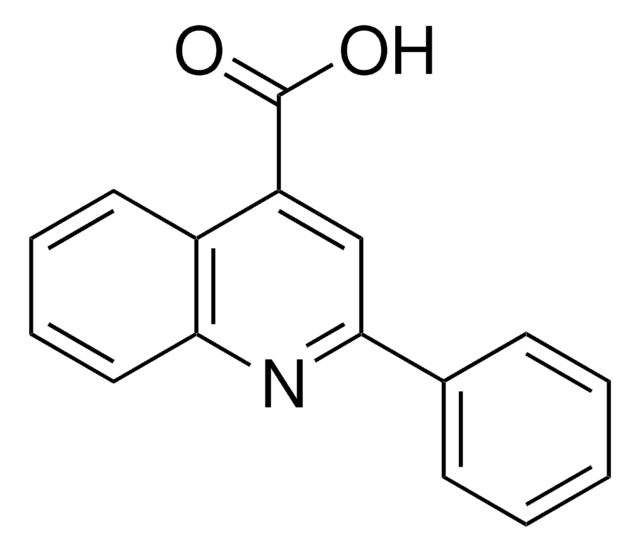
SMILES記法
OC(=O)c1ccnc2ccccc12
InChI
1S/C10H7NO2/c12-10(13)8-5-6-11-9-4-2-1-3-7(8)9/h1-6H,(H,12,13)
InChI Key
VQMSRUREDGBWKT-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
4-Quinolinecarboxylic acid was used in the coupling reaction with diamine linker. A 4-quinolinecarboxylic acid analogue, brequinar sodium was used to inhibit dihydroorotate dehydrogenase and the de novo biosynthesis of pyrimidine.
生物化学的/生理学的作用
4-Quinolinecarboxylic acid showed anti-tumor activity against L1210 leukemia and B16 melanoma.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
ターゲットの組織
Respiratory system
保管分類コード
11 - Combustible Solids
WGK
WGK 3
引火点(°F)
Not applicable
引火点(℃)
Not applicable
個人用保護具 (PPE)
dust mask type N95 (US), Eyeshields, Gloves
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
Jan Code
174823-BULK:
174823-1G:
174823-VAR:
174823-5G:
174823-250MG:
D L Dexter et al.
Cancer research, 45(11 Pt 1), 5563-5568 (1985-11-01)
A novel, substituted 4-quinolinecarboxylic acid (NSC 339768) demonstrated antitumor activity against L1210 leukemia and B16 melanoma in the National Cancer Institute's Developmental Therapeutics Program. An extensive analogue synthesis program was initiated; over 200 derivatives were synthesized and tested for anticancer
[A case of Gaucher's disease treated with hydroxyphenylcinchoninic acid].
P DANIEL MARTINEZ et al.
Boletin medico del Hospital Infantil de Mexico, 8(2), 189-194 (1951-04-01)
A J Dobson et al.
Acta crystallographica. Section C, Crystal structure communications, 55 ( Pt 7), 1192-1195 (1999-08-13)
The previously undescribed title substance, C10H7NO2.-3H2O, crystallized in the centrosymmetric space group P1 with one zwitterionic organic molecule and three water molecules in the asymmetric unit. One N-H...O and six O-H...O hydrogen bonds are present in this structure, with donor-acceptor
Murugesan Dinakaran et al.
Medicinal chemistry (Shariqah (United Arab Emirates)), 4(5), 482-491 (2008-09-11)
Thirty four novel 7-fluoro/nitro-1,2-dihydro-5-oxo-8-(sub)-5H-thiazolo[3,2-a]quinoline-4-carboxylic acids were synthesized from 2,4-dichlorobenzoic acid and 2,4-dichloro-5-fluoroacetophenone by multi step reaction, evaluated for in vitro and in vivo antimycobacterial activities against Mycobacterium tuberculosis H37Rv (MTB), multi-drug resistant Mycobacterium tuberculosis (MDR-TB) and Mycobacterium smegmatis (MC2) and
He Huang et al.
The Journal of organic chemistry, 74(15), 5476-5480 (2009-07-04)
We developed a simple and convenient copper-catalyzed method for the synthesis of quinoline-2-carboxylate derivatives through sequential intermolecular addition of alkynes onto imines and subsequent intramolecular ring closure by arylation. The efficiency of this system allowed the reactions to be carried
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)