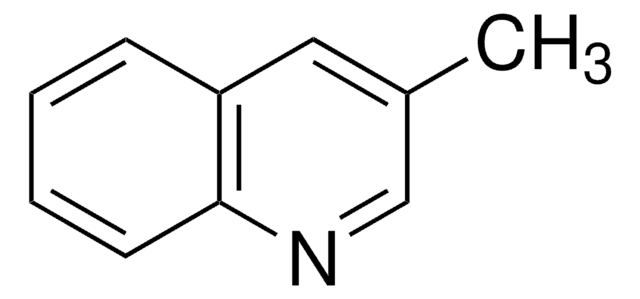
おすすめの製品
蒸気密度
>1 (vs air)
アッセイ
98%
屈折率
n20/D 1.614 (lit.)
bp
256-260 °C (lit.)
密度
1.067 g/mL at 20 °C (lit.)
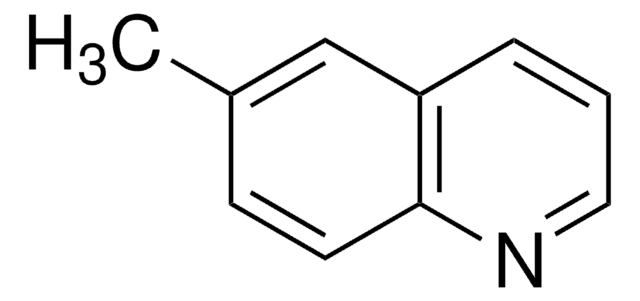
SMILES記法
Cc1ccc2ncccc2c1
InChI
1S/C10H9N/c1-8-4-5-10-9(7-8)3-2-6-11-10/h2-7H,1H3
InChI Key
LUYISICIYVKBTA-UHFFFAOYSA-N
類似した製品をお探しですか? 訪問 製品比較ガイド
アプリケーション
6-Methylquinoline can be used as primary carbon source in culture of Pseudomonas putida QP1. 6-Methylquinoline was used in the synthesis of fluorescent halide-sensitive quinolinium dyes and fluorescent probes for determination of chloride in biological systems.
生物化学的/生理学的作用
6-Methylquinoline undergoes biodegradation by quinoline-degrading culture of Pseudomonas putida.
シグナルワード
Warning
危険有害性情報
危険有害性の分類
Acute Tox. 4 Oral - Skin Irrit. 2
保管分類コード
10 - Combustible liquids
WGK
WGK 3
引火点(°F)
235.4 °F - closed cup
引火点(℃)
113 °C - closed cup
個人用保護具 (PPE)
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
適用法令
試験研究用途を考慮した関連法令を主に挙げております。化学物質以外については、一部の情報のみ提供しています。 製品を安全かつ合法的に使用することは、使用者の義務です。最新情報により修正される場合があります。WEBの反映には時間を要することがあるため、適宜SDSをご参照ください。
消防法
第4類:引火性液体
第三石油類
危険等級III
非水溶性液体
Jan Code
108928-5KG:
108928-VAR:
108928-BULK:
108928-25G:
108928-100G:
Chloride sensitive probes for biological applications.
Geddes CD, et al.
Dyes and Pigments, 48(3), 227-231 (2001)
S Rothenburger et al.
Applied and environmental microbiology, 59(7), 2139-2144 (1993-07-01)
Selective culturing of pseudomonads that could degrade quinoline led to enrichment cultures and pure cultures with expanded substrate utilization and transformation capabilities for substituted quinolines in immobilized and batch cultures. Immobilized cells of the pseudomonad cultures rapidly transformed quinolines to
C D Geddes et al.
Analytical biochemistry, 293(1), 60-66 (2001-05-25)
Three fluorescent halide-sensitive quinolinium dyes have been produced by the reaction of the 6-methylquinoline heterocyclic nitrogen base with methyl bromide, methyl iodide, and 3-bromo-1-propanol. The quaternary salts, unlike the precursor molecule, are readily water soluble and the fluorescence intensity of
Umar Farooq Rizvi et al.
Acta crystallographica. Section C, Crystal structure communications, 64(Pt 10), o547-o549 (2008-10-08)
Molecules of (E)-3-(2-chloro-6-methylquinolin-3-yl)-1-(5-iodo-2-thienyl)prop-2-en-1-one, C(17)H(11)ClINOS, (I), and (E)-3-(2-chloro-6-methylquinolin-3-yl)-1-(5-methyl-2-furyl)prop-2-en-1-one, C(18)H(14)ClNO(2), (II), adopt conformations slightly twisted from coplanarity. Both structures are devoid of classical hydrogen bonds. However, nonclassical C-H...O/N interactions [with C...O = 3.146 (5) A and C...N = 3.487 (3) A] link
C E Scharping et al.
Carcinogenesis, 14(5), 1041-1047 (1993-05-01)
The hepatic microsomal metabolism of the carcinogenic 8-methylquinoline (8MQ) and its noncarcinogenic isomer, 6-methylquinoline (6MQ), were compared for preparations from control rats and rats pretreated with phenobarbital or 3-methylcholanthrene. For each compound the alcohol was the major metabolite, constituting 50-75%
ライフサイエンス、有機合成、材料科学、クロマトグラフィー、分析など、あらゆる分野の研究に経験のあるメンバーがおります。.
製品に関するお問い合わせはこちら(テクニカルサービス)