1044800
USP
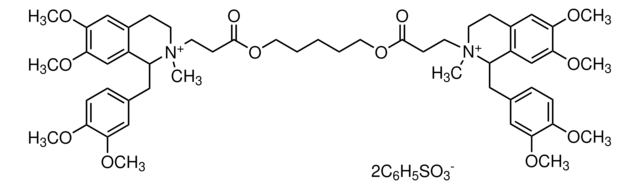
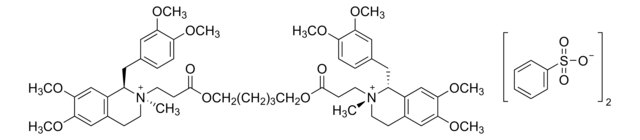
Atracurium besylate
United States Pharmacopeia (USP) Reference Standard
Sinonimo/i:
2,2′-[1,5-Pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methylisoquinolinium]dibenzenesulfonate, BW-33A, Tracrium
About This Item
Prodotti consigliati
Grado
pharmaceutical primary standard
Famiglia di API
atracurium
Produttore/marchio commerciale
USP
applicazioni
pharmaceutical (small molecule)
Formato
neat
Temperatura di conservazione
2-8°C
Stringa SMILE
[O-]S(=O)(=O)c1ccccc1.[O-]S(=O)(=O)c2ccccc2.COc3ccc(CC4c5cc(OC)c(OC)cc5CC[N+]4(C)CCC(=O)OCCCCCOC(=O)CC[N+]6(C)CCc7cc(OC)c(OC)cc7C6Cc8ccc(OC)c(OC)c8)cc3OC
InChI
1S/C53H72N2O12.2C6H6O3S/c1-54(22-18-38-32-48(62-7)50(64-9)34-40(38)42(54)28-36-14-16-44(58-3)46(30-36)60-5)24-20-52(56)66-26-12-11-13-27-67-53(57)21-25-55(2)23-19-39-33-49(63-8)51(65-10)35-41(39)43(55)29-37-15-17-45(59-4)47(31-37)61-6;2*7-10(8,9)6-4-2-1-3-5-6/h14-17,30-35,42-43H,11-13,18-29H2,1-10H3;2*1-5H,(H,7,8,9)/q+2;;/p-2
XXZSQOVSEBAPGS-UHFFFAOYSA-L
Informazioni sul gene
human ... CHRNA1(1134) , CHRNB1(1140) , CHRND(1144) , CHRNE(1145) , CHRNG(1146)
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- The feasibility of the posterior tibial nerve-flexor hallucis brevis pathway applied in neuromuscular monitoring: This multicentric, controlled, and prospective clinical trial investigates a new method for neuromuscular monitoring using Atracurium besylate, providing insights into improving surgical anesthesia practices (Chen et al., 2024).
- Onset and duration of action of escalating doses of atracurium in anesthetized healthy goats: This study assesses the pharmacokinetics and pharmacodynamics of Atracurium besylate in veterinary medicine, highlighting its efficacy and safety in non-human subjects, which is crucial for translating findings to human clinical settings (Ishihara et al., 2024).
- Under-dosing and over-dosing of neuromuscular blocking drugs and reversal agents: beware of the risks: This editorial discusses the critical balance required in dosing Atracurium besylate to optimize patient outcomes and minimize side effects in surgical settings, emphasizing the importance of precise dosing for effective neuromuscular blockade (Hunter and Blobner, 2024).
- Comparison of the Onset of Action, Maintenance, and Recovery of Three Weight-based Dosing of Cisatracurium in Patients with Morbid Obesity in Laparoscopic Bariatric Surgery: Although focused on Cisatracurium, this study provides comparative insights that could be applicable to similar studies on Atracurium besylate, particularly in terms of dose adjustment for specific patient populations undergoing surgical procedures (Rokhtabnak et al., 2023).
- Neuromuscular Blockade: This piece provides a broad overview of neuromuscular blocking agents like Atracurium besylate, discussing their mechanisms and applications in clinical anesthesia, which is essential for understanding the full scope of these drugs′ utility in medical practice (Cook and Simons, 2024).
Azioni biochim/fisiol
Risultati analitici
Altre note
Prodotti correlati
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Certificati d'analisi (COA)
Ci dispiace, ma al momento non ci sono COA disponibili online per questo prodotto.
Se ti serve aiuto, non esitare a contattarci Servizio Clienti
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.