T4014
Tobramicina
Aminoglycoside antibiotic
Sinonimo/i:
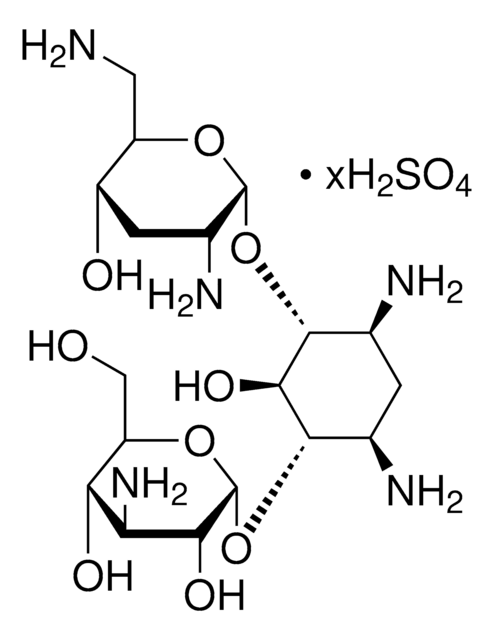
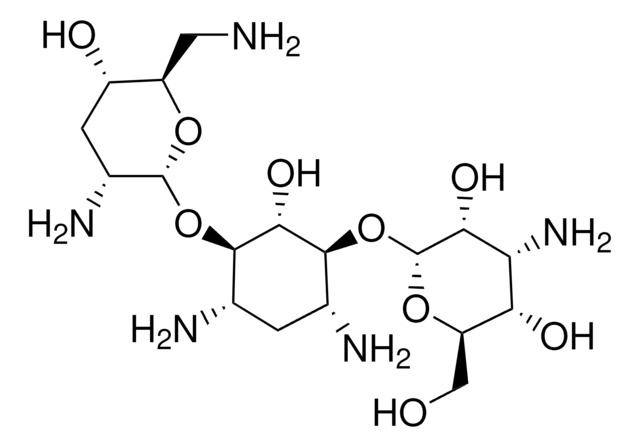
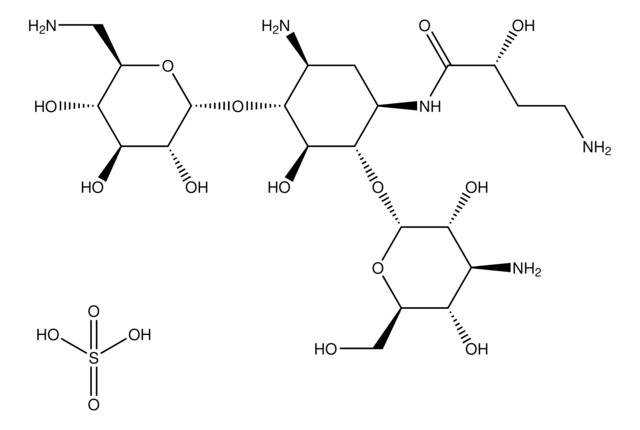
Nebramicina Fattore 6, O-[3-amino-3-deossi-α-D-glucopiranosil-(1→6)]-O-[2,6-diamino-2,3,6-trideossi-α-D-riboesopiranosil-(1→4)]-2-deossi-D-streptamina
About This Item
Prodotti consigliati
Origine biologica
Streptomyces tenebrarius
Livello qualitativo
Stato
powder
Condizioni di stoccaggio
(Keep container tightly closed in a dry and well-ventilated place. Keep in a dry place.)
Concentrazione
≥900 μg/mg (anhydrous)
Colore
white to off-white
Spettro attività antibiotica
Gram-negative bacteria
Modalità d’azione
protein synthesis | interferes
Temperatura di conservazione
2-8°C
Stringa SMILE
NC[C@H]1O[C@H](O[C@@H]2[C@@H](N)C[C@@H](N)[C@H](O[C@H]3O[C@H](CO)[C@@H](O)[C@@H](N)[C@H]3O)[C@H]2O)[C@H](N)C[C@@H]1O
InChI
1S/C18H37N5O9/c19-3-9-8(25)2-7(22)17(29-9)31-15-5(20)1-6(21)16(14(15)28)32-18-13(27)11(23)12(26)10(4-24)30-18/h5-18,24-28H,1-4,19-23H2/t5-,6+,7+,8-,9+,10+,11+,12+,13+,14-,15+,16-,17+,18+/m0/s1
NLVFBUXFDBBNBW-SNGYORCQSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- to study the effect of tobramycin on bacteriophage infected biofilms
- to study the effects of antibiotics on Pseudomonas aeruginosa strain
- as a comparator antibiotic
Azioni biochim/fisiol
Modalità d′azione: Si lega alla subunità ribosomiale 70S, inibisce la traslocazione e suscita errori di codifica.
Spettro di attività: Batteri gram negativi. Non efficace nei confronti di Enterococci.
Tobramycin is used to treat Pseudomonas aeruginosa lung infections and is used in combination with other antibiotics to treat urinary tract infections, gynecologic infections, peritonitis, endocarditis, pneumonia, sepsis, respiratory infections, osteomyelitis, and other soft-tissue infections. It is a potential therapy for sinus infections.
Altre note
Avvertenze
Warning
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Repr. 2
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.