P5931
Phosphatase, Alkaline from Escherichia coli
lyophilized powder, 30-60 units/mg protein (in glycine buffer)
Sinonimo/i:
Orthophosphoric-monoester phosphohydrolase (alkaline optimum)
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Prodotti consigliati
Stato
lyophilized powder
Livello qualitativo
Attività specifica
30-60 units/mg protein (in glycine buffer)
Composizione
Protein, ~50% biuret
Temperatura di conservazione
−20°C
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Alkaline phosphatase (ALP) of E.coli is a member of an enzyme group that possesses intragenic complementation.
Applicazioni
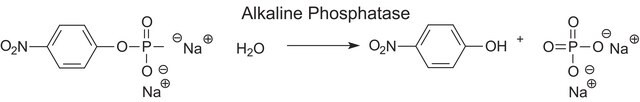
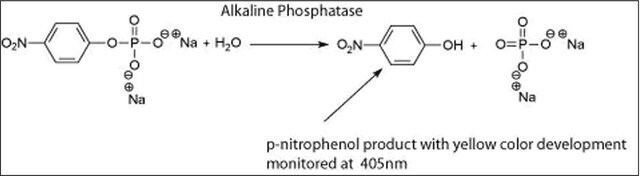
Alkaline phosphatase is used for conjugation to antibodies and other proteins for ELISA, Western blotting, and histochemical detection. It may be used for protein labeling when high sensitivity is required. Product P5931 has been used during immunoblots to treat cell membranes prior to Tau1 incubation.
The enzyme from Sigma has been used to develop a synthetic nanopore membrane. This membrane mimics protein channels that are regulated by phosphorylation/dephosphorylation and uses an aligned array of carbon nanotubes (CNTs) impregnated in a polystyrene matrix.
Azioni biochim/fisiol
Alkaline phosphatase, from Escherichia coli, is a dimeric, non-glycosylated protein which mainly reside in the periplasmic space. Three known isoforms exist. The enzyme requires zinc, and is activated by magnesium. E. coli akaline phosphatase has a broad specificity for phosphate esters.
The enzyme is a phosphohydrolase having an optimal pH of 10 in vitro. The actual optimum pH varies depending on the nature and concentration of the substrate, the type of buffer, the phosphate acceptor, and to some extent the nature of the isoenzymes. It catalyzes the hydrolysis of phosphate monoesters such as p-nitrophenyl phosphate, phenyl phosphate, phenolphthalein phosphate, α-glycerol phosphate, β-glycerol phosphate, 2-phosphorylglycerate, triosephosphate, glucose-6-phosphate, glucose 1-phosphate, fructose 1-phosphate, fructose 6-phosphate, adenosine 5-phosphate adenosine 3-phosphate, phosphoenolpyruvate, and β-nicotinamide adenine dinucleotide phosphate. The activity is inhibited by 1,10-phenanthroline monohydrate, diethylenetriaminepentaacetic acid, ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid, and ethylenediaminetetraacetic acid disodium salt dihydrate.
Definizione di unità
One unit will hydrolyze 1.0 μmole of p-nitrophenyl phosphate per min at pH 10.4 at 37 °C.
Stato fisico
Lyophilized powder containing Tris buffer salts, MgSO4, and ZnSO4
Nota sulla preparazione
Chromatographically purified
Inibitore
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
1.Reid, T., and Wilson
E. coli Alkaline Phosphatase,The Enzymes, 3rd Ed. Vol. 4, 4, 373-373 (1971)
Characterization of Heterodimeric Alkaline Phosphatases from Escherichia coli: An Investigation of Intragenic Complementation
Hehir MJ, et al.
Journal of molecular biology, 304(4), 645-656 (2000)
A Takeda et al.
The Journal of biological chemistry, 275(8), 5395-5399 (2000-02-22)
Increased expression of heme oxygenase-1 (HO-1) is a common feature in a number of neurodegenerative diseases. Interestingly, the spatial distribution of HO-1 expression in diseased brain is essentially identical to that of pathological expression of tau. In this study, we
Alkaline phosphatase from Thermotoga neapolitana.
A Savchenko et al.
Methods in enzymology, 331, 298-305 (2001-03-27)
R A Anderson et al.
Proceedings of the National Academy of Sciences of the United States of America, 72(1), 394-397 (1975-01-01)
To facilitate the study of individual metal binding sites of polymeric metalloproteins, conversion of exchange-labile Co(II) in E. coli alkaline phosphatase (EC 3.1.3.1) to exchange-inert Co(III) was examined. Oxidation of Co(II) alkaline phosphatase with hydrogen peroxide results in a single
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.