L2037
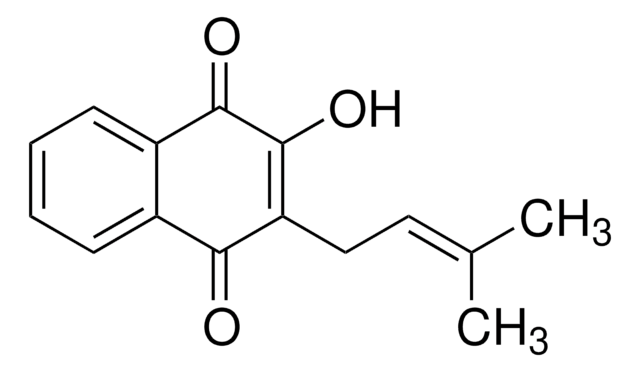
β-Lapachone
≥98% (TLC)
Sinonimo/i:
ARQ 501, NSC 26326, NSC 629749, SL 11001
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
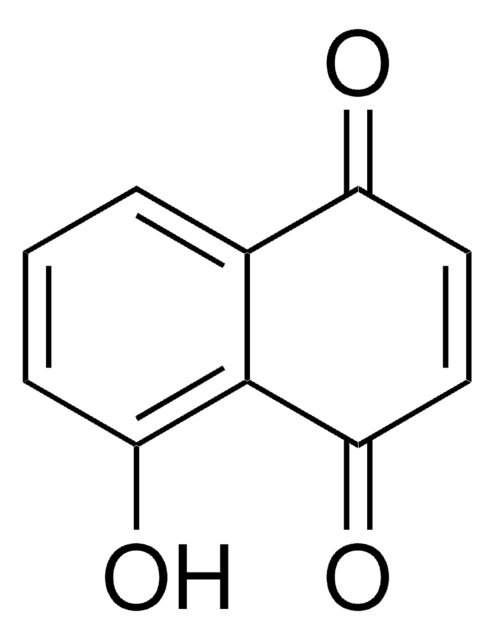
Formula empirica (notazione di Hill):
C15H14O3
Numero CAS:
Peso molecolare:
242.27
Numero MDL:
Codice UNSPSC:
12352200
ID PubChem:
NACRES:
NA.77
Prodotti consigliati
Origine biologica
synthetic (organic)
Saggio
≥98% (TLC)
Stato
powder
Stringa SMILE
CC1(C)CCC2=C(O1)c3ccccc3C(=O)C2=O
InChI
1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3
QZPQTZZNNJUOLS-UHFFFAOYSA-N
Applicazioni
β-Lapachone has been used:
- as an anticancer compound in catalase-inhibitable luminol/hydrogen peroxide (HRP)-dependent chemiluminometric assay in Lewis lung carcinoma (LLC) cells and isolated mitochondria
- as a naphthoquinone to study its effects on the growth and differentiation of mice granulocyte and macrophage progenitor cells
- as a substrate to study the enzyme activity of human recombinant NAD(P)H dehydrogenase 1 (NQO1) protein
Azioni biochim/fisiol
β-Lapachone acts as a DNA topoisomerase type I inhibitor. It exhibits anti-fungal, anti-bacterial, trypanocidal, and antiviral properties. β-Lapachone also inhibits nitric oxide (NO) and inducible NO synthase (iNOS) in alveolar macrophages.
β-Lapachone is a naturally occurring quinone obtained from the bark of the lapacho tree (Tabebuia avellanedae) with cancer chemopreventive properties. Induces apoptosis in HL-60 and human prostate cancer cells.
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
David Siegel et al.
Biochemical pharmacology, 83(8), 1033-1040 (2012-01-03)
Quinones represent a large and diverse class of antitumor drugs and many quinones are approved for clinical use or are currently undergoing evaluation in clinical trials. For many quinones reduction to the hydroquinone has been shown to play a key
S M Planchon et al.
Cancer research, 55(17), 3706-3711 (1995-09-01)
beta-Lapachone and certain of its derivatives directly bind and inhibit topoisomerase I (Topo I) DNA unwinding activity and form DNA-Topo I complexes, which are not resolvable by SDS-K+ assays. We show that beta-lapachone can induce apoptosis in certain cells, such
Oliver Quevedo et al.
Chemical research in toxicology, 24(12), 2106-2108 (2011-11-19)
β-Lapachone (β-lap) is a promising antitumoral agent. DNA base oxidation and alkylation are among the expected damages by β-lap. Herein, we have explored the role that the homologous recombination pathway (HR), a critical DNA repair process in Saccharomyces cerevisiae, has
Xiumei Huang et al.
Cancer research, 72(12), 3038-3047 (2012-04-26)
Agents, such as β-lapachone, that target the redox enzyme, NAD(P)H:quinone oxidoreductase 1 (NQO1), to induce programmed necrosis in solid tumors have shown great promise, but more potent tumor-selective compounds are needed. Here, we report that deoxynyboquinone kills a wide spectrum
Isabella M F Cavalcanti et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 44(3), 332-340 (2011-09-06)
The aim of this study was to encapsulate lapachone (β-lap) or inclusion complex (β-lap:HPβ-CD) in liposomes and to evaluate their physicochemical characteristics. In addition, the investigation of the main aspects of the interaction between β-lap and 2-hydroxypropyl-β-cyclodextrin (HPβ-CD), using both
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.