A9384
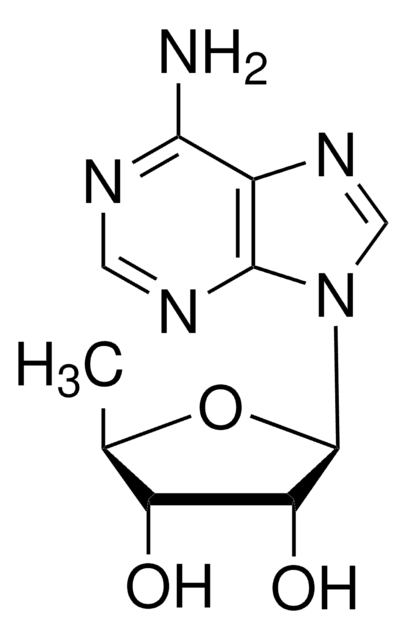
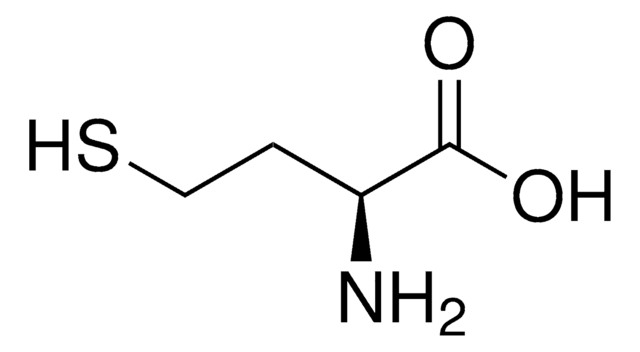
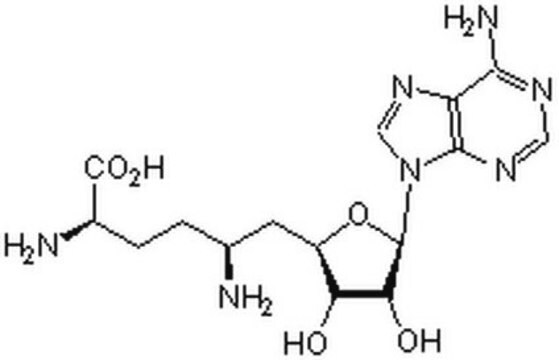
S-(5′-Adenosyl)-L-homocysteine
crystalline
Sinonimo/i:
5′-Deoxy-S-adenosyl-L-homocysteine, AdoHcy, S-(5′-Deoxyadenosine-5′)-L-homocysteine
About This Item
Prodotti consigliati
Saggio
≥98.0% (HPLC)
≥98.0% (TLC)
Livello qualitativo
Stato
crystalline
PM
384.41
Solubilità
1 M HCl: soluble 19.60-20.40 mg/mL, clear to slightly hazy, colorless to faintly yellow
Temperatura di conservazione
−20°C
Stringa SMILE
N[C@@H](CCSC[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23)C(O)=O
InChI
1S/C14H20N6O5S/c15-6(14(23)24)1-2-26-3-7-9(21)10(22)13(25-7)20-5-19-8-11(16)17-4-18-12(8)20/h4-7,9-10,13,21-22H,1-3,15H2,(H,23,24)(H2,16,17,18)/t6-,7+,9+,10+,13+/m0/s1
ZJUKTBDSGOFHSH-WFMPWKQPSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Applicazioni
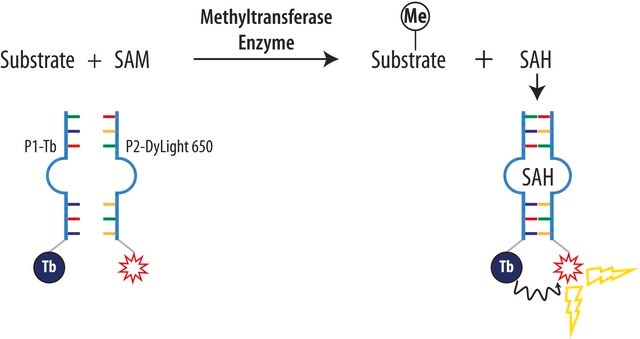
- to investigate whether AdoHcy competes with AdoMet in the down-regulation of reporter activity of LUC reporter gene
- as a reagent to study the abundance patterns of SAH and its correlation with vertebrate metamorphosis
- in the optimization of the protein (lysine K) methyltransferase SET7/9 activity assay
- in the fluorescence polarization (FP) assay during dengue virus methyltransferase activity measurement
- as a standard for the measurement of SAH from blood samples by high performance liquid chromatography (HPLC) with fluorimetric detection method
Azioni biochim/fisiol
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 3
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Dispositivi di protezione individuale
Eyeshields, Gloves, type N95 (US)
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Epigenetic modifications are thought to occur through two key interconnected processes—DNA methylation and the covalent modification of histones.
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.