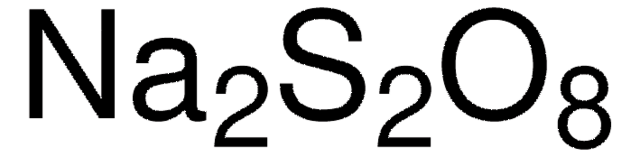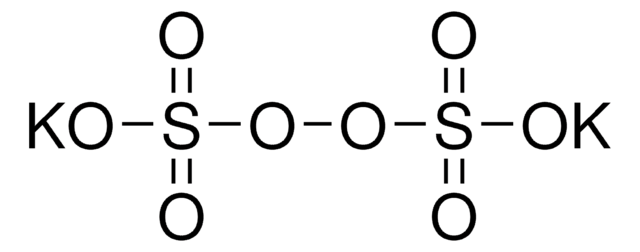216232
Sodium persulfate
reagent grade, ≥98%
Sinonimo/i:
Sodium peroxodisulfate
About This Item
Prodotti consigliati
Grado
reagent grade
Livello qualitativo
Saggio
≥98%
Stato
powder, crystals or granules
Impiego in reazioni chimiche
reagent type: oxidant
Stringa SMILE
[Na+].[Na+].[O-]S(=O)(=O)OOS([O-])(=O)=O
InChI
1S/2Na.H2O8S2/c;;1-9(2,3)7-8-10(4,5)6/h;;(H,1,2,3)(H,4,5,6)/q2*+1;/p-2
CHQMHPLRPQMAMX-UHFFFAOYSA-L
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Descrizione generale
Applicazioni
- For the radical cyclization cascade reaction of 2-alkynylbenzonitriles and sodium arylsulfinates to synthesize sulfonated indenones.
- In the silica-supported aluminum chloride-catalyzed Baeyer-Villiger oxidation of cyclic and acyclic ketones to lactones or esters.
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Ox. Sol. 3 - Resp. Sens. 1 - Skin Irrit. 2 - Skin Sens. 1 - STOT SE 3
Organi bersaglio
Respiratory system
Codice della classe di stoccaggio
5.1B - Oxidizing hazardous materials
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
Not applicable
Punto d’infiammabilità (°C)
Not applicable
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.