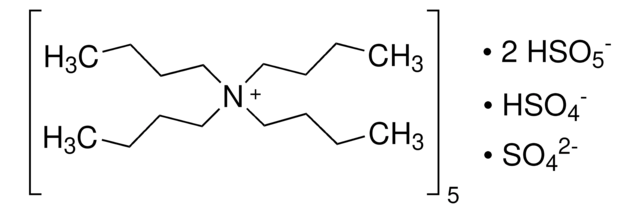
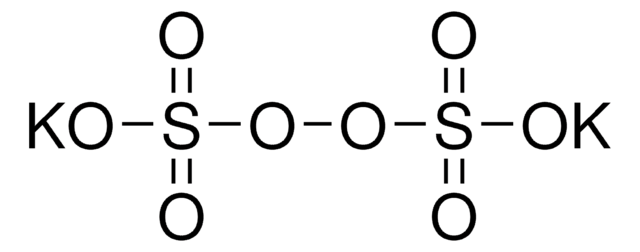228036
OXONE®, monopersulfate compound
Sinonimo/i:
Potassium peroxymonosulfate
About This Item
Prodotti consigliati
Tensione di vapore
<0.0000017 hPa
Livello qualitativo
Forma fisica
powder
Impiego in reazioni chimiche
reagent type: oxidant
Concentrazione
>4.0% (active oxygen basis (by Na2S2O3, titration))
pH
2.1 (77 °C, 30 g/L)
Stringa SMILE
[K+].[K+].[K+].[K+].[K+].OS([O-])(=O)=O.[O-]S([O-])(=O)=O.O[S+]([O-])([O-])(=O)=O.O[S+]([O-])([O-])(=O)=O
InChI
1S/5K.2H2O5S.2H2O4S/c;;;;;2*1-5-6(2,3)4;2*1-5(2,3)4/h;;;;;2*1H,(H,2,3,4);2*(H2,1,2,3,4)/q5*+1;;;;/p-5
HJKYXKSLRZKNSI-UHFFFAOYSA-I
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Applicazioni
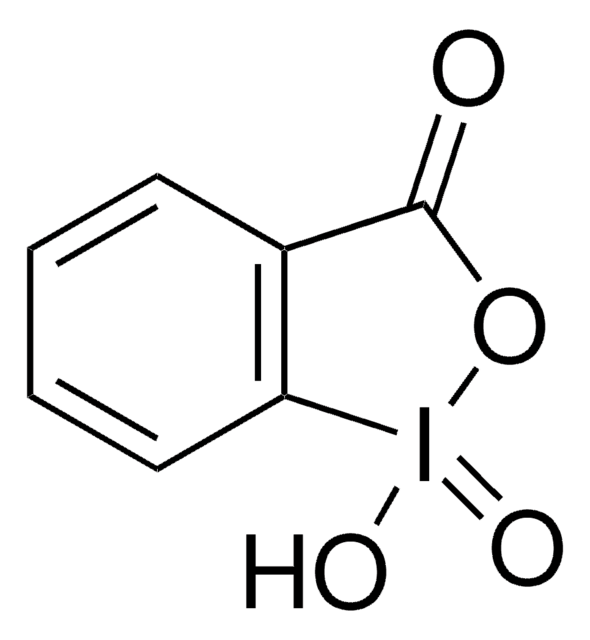
2-Iodoxybenzenesulfonic Acid as an Extremely Active Catalyst for the Selective Oxidation of Alcohols to Aldehydes, Ketones, Carboxylic Acids, and Enones with Oxone
A Convenient Halogenation of α,β-Unsaturated Carbonyl Compounds with OXONE® and Hydrohalic Acid (HBr, HCl)
Note legali
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Acute Tox. 4 Oral - Aquatic Chronic 2 - Eye Dam. 1 - Skin Corr. 1B
Classe di pericolosità dell'acqua (WGK)
WGK 3
Dispositivi di protezione individuale
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Articoli
Oxidation and reduction reactions are some of the most common transformations encountered in organic synthesis
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.