P39303
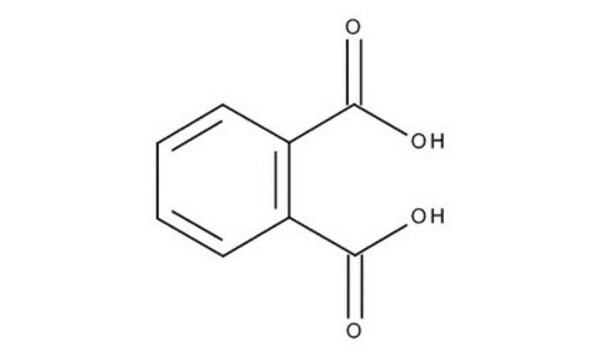
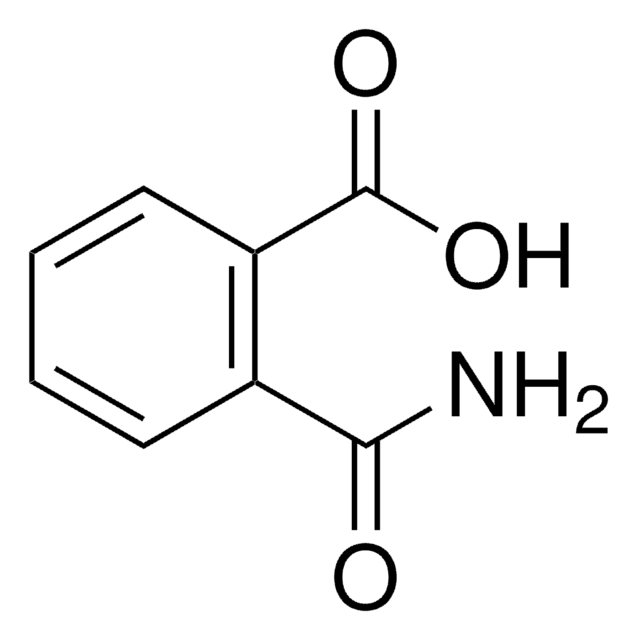
Phthalic acid
reagent grade, 98%
Sinonimo/i:
1,2-Benzenedicarboxylic acid
Autenticatiper visualizzare i prezzi riservati alla tua organizzazione & contrattuali
About This Item
Formula condensata:
C6H4-1,2-(CO2H)2
Numero CAS:
Peso molecolare:
166.13
Beilstein:
608199
Numero CE:
Numero MDL:
Codice UNSPSC:
12352100
ID PubChem:
NACRES:
NA.21
Prodotti consigliati
Grado
reagent grade
Livello qualitativo
Saggio
98%
Forma fisica
crystalline powder
powder
Punto di fusione
210-211 °C (dec.) (lit.)
Stringa SMILE
OC(C1=C(C(O)=O)C=CC=C1)=O
InChI
1S/C8H6O4/c9-7(10)5-3-1-2-4-6(5)8(11)12/h1-4H,(H,9,10)(H,11,12)
XNGIFLGASWRNHJ-UHFFFAOYSA-N
Cerchi prodotti simili? Visita Guida al confronto tra prodotti
Categorie correlate
Descrizione generale
Phthalic acid (PA) is the final metabolite of phthalic acid esters (PAEs). Its interaction with well-ordered MgO(100) (magnesium oxide) thin films has been investigated. The efficiency of activated carbon suspended in aqueous medium for the adsorption of PA has been analyzed. The degradation of PA via oxidation by electro-Fenton (EF) and solar photoelectro-Fenton (SPEF) has been reported.
Applicazioni
Phthalic acid may be used as an additive to the mobile phase to enhance the detection sensitivity of amino acids (AAs) by hydrophilic interaction liquid chromatography (HILIC) coupled with electrospray ionization tandem mass spectrometry (ESI-MS/MS).
Avvertenze
Danger
Indicazioni di pericolo
Consigli di prudenza
Classi di pericolo
Eye Dam. 1
Codice della classe di stoccaggio
11 - Combustible Solids
Classe di pericolosità dell'acqua (WGK)
WGK 1
Punto d’infiammabilità (°F)
334.4 °F
Punto d’infiammabilità (°C)
168 °C
Dispositivi di protezione individuale
dust mask type N95 (US), Eyeshields, Gloves
Scegli una delle versioni più recenti:
Possiedi già questo prodotto?
I documenti relativi ai prodotti acquistati recentemente sono disponibili nell’Archivio dei documenti.
I clienti hanno visto anche
Adsorption/bioadsorption of phthalic acid, an organic micropollutant present in landfill leachates, on activated carbons.
Mendez-Diaz JD, et al.
Journal of Colloid and Interface Science, 369(1), 358-365 (2012)
Benzoic Acid and Phthalic Acid on Atomically Well-Defined MgO(100) Thin Films: Adsorption, Interface Reaction, and Thin Film Growth.
Xu T, et al.
The Journal of Physical Chemistry C, 119(48), 26968-26979 (2015)
Mineralization of phthalic acid by solar photoelectro-Fenton with a stirred boron-doped diamond/air-diffusion tank reactor: Influence of Fe3+ and Cu2+ catalysts and identification of oxidation products.
Garcia-Segura S, et al.
Electrochimica Acta, 113, 609-619 (2013)
Du Yeon Bang et al.
Toxicological research, 27(4), 191-203 (2011-12-01)
There has been growing concern about the toxicity of phthalate esters. Phthalate esters are being used widely for the production of perfume, nail varnish, hairsprays and other personal/cosmetic uses. Recently, exposure to phthalates has been assessed by analyzing urine for
Wanshu Qi et al.
Analytica chimica acta, 870, 75-82 (2015-03-31)
In this work, 0.08 mmol L(-1) of phthalic acid was introduced as a mobile phase additive to quantify free amino acids (AAs) by hydrophilic interaction liquid chromatography (HILIC) coupled with electrospray ionization tandem mass spectrometry (ESI-MS/MS). The addition of phthalic
Il team dei nostri ricercatori vanta grande esperienza in tutte le aree della ricerca quali Life Science, scienza dei materiali, sintesi chimica, cromatografia, discipline analitiche, ecc..
Contatta l'Assistenza Tecnica.